Continued from previous page.
Hale's "Third Alkaloid of Hydrastis" - Table Showing the Examination of Forty-nine Specimens of Commercial Powdered Hydrastis
HALE'S "THIRD ALKALOID OF HYDRASTIS."—History.—This substance is recorded under the name "Hale's Third Alkaloid." While it is true that Mr. A. K. Hale [American Journal of Pharmacy, 1873, p. 247.] obtained a body from hydrastis that seemingly possessed properties that would distinguish it from both berberine and hydrastine, he did not really announce that it was a new alkaloid, as some persons seem to suppose. The heading of his paper, "Is there a Third Alkaloid in Hydrastis Canadensis," indicates that the author was undecided, and took this method to bring his experiments before the public, in order that subsequent investigators might determine the matter.
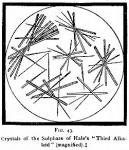 [Reproduced from American Journal of Pharmacy, Nov. 1875, as represented by Mr. John C. Burt.]
[Reproduced from American Journal of Pharmacy, Nov. 1875, as represented by Mr. John C. Burt.]
The material obtained by Mr. Hale was afterward identified by Mr. John C. Burt, [American Journal of Pharmacy, 1875, p. 481.] who gave a number of additional reactions. He also presented a microdrawing of the sulphate, which we reproduce herewith (Fig. 43). Finally it was obtained by Mr. Herman Lerchen, [American Journal of Pharmacy, 1878, p. 470.] who affixed to it the name xanthopuccine.
It would seem that these determinations should establish the fact that such an alkaloid existed, but we can not pass the matter without presenting evidence that we feel is worthy of consideration, and which leads us to view the impure substance obtained by these gentlemen as a mixture of berberine, hydrastine, and impurities of hydrastis. We will first review Mr. Hale's process, as follows: "I treated the powdered root of Hydrastis canadensis in a percolator with distilled water until the strength seemed to be exhausted; then I proceeded to remove the berberine as a hydrochlorate by the addition of hydrochloric acid. Removing this precipitate of hydrochlorate of berberine by filtration, I then proceeded to obtain the hydrastine by adding water of ammonia (10 per cent.) until a precipitate ceased to be thrown down. This precipitate I separated by filtration, and dissolved in and crystallized from alcohol, when, instead of hydrastine, as the books described it, I found that the characteristic prisms of hydrastine were colored by and intimately mixed with a yellow powder, which I supposed to be berberine that had not been thrown down as a hydrochlorate. Being thus a little disconcerted at not obtaining the result I hoped for, I made another percolate of the drug, and to the mother liquor of berberine I carefully added water of ammonia (10 per cent.) to the neutral point. The precipitate thus obtained I dissolved in and crystallized from alcohol, which furnished beautiful and well-defined prismatic crystals of hydrastine, free from yellow coloring matter at all resembling berberine.
"To the neutral mother liquor of hydrastine, I now added water of ammonia (10 per cent.) to a strong alkaline reaction. This gave me a yellow precipitate, which I separated, and found to correspond with the yellow powder above mentioned as accompanying the first attempt to obtain hydrastine, and to be darker in color than berberine."
Mr. Hale thought that this yellow substance might be a new alkaloid, and it has since been referred to as "Hale's Third Alkaloid."
Remarks.—An important point in the foregoing paper is the oversight made in neglecting to state whether the hydrochloric acid was added to the percolate until it was in strong excess (see p. 113). [Mr. Lerchen, it is true, used the expression, "acidulating it strongly with hydrochloric acid," but he might have considered a decided acid reaction towards litmus as sufficient. In our experience, in order to precipitate all of the berberine possible (and it can not all be thrown down), hydrochloric acid to the extent of one-fourth the bulk of the percolate should be used.]
If it is only added until a decided acid reaction ensues, the natural combination in which the berberine exists is but partially overcome, and a large amount of berberine remains in solution. This is a feature that manufacturers of these alkaloids have to guard against, for if a considerable proportion of berberine is left in the mother liquid, it is largely thrown down with the hydrastine, and after being associated in this manner, its removal is difficult (see p. 134). Hence it follows that, if this precaution is not observed, the second precipitate by Mr. Hale's method is exactly as he describes it, but the yellow substance, as we have every reason to believe, is impure berberine.
The experimentor must also not overlook the fact that the alkaloids, hydrastine and berberine, are not the only substances thrown from solution by the excess of ammonia. A dark-colored, resinous body is also separated, and it adheres with some tenacity to the precipitate. [This substance is of considerable general interest, but it is not desirable to study it in this paper. Prof. E. Scheffer made an interesting line of experiments with it some years ago, and communicated his observations to us, but they have not been completed.] Thus it follows that, according to our views, also, and according to the results of our experience, a yellow precipitate may be obtained by means of Mr. Hale's process. Before introducing further testimony that we have to offer concerning the nature of this precipitate, we shall call particular attention to the following points:
1st. When ammonia water is added to the percolate until this liquid is neutral, a portion only of the hydrastine precipitates. This percolate contains salts of calcium and aluminium (doubtless from adhering soil), and especially is the hydroxide of aluminium thrown down before the hydrastine, or with the first portions of it. Therefore it may happen that, by exercising care in neutralization, the larger share of the hydrastine may really remain in solution after the liquid ceases to affect litmus paper. The filtration of such a neutral liquid, and addition of excess of ammonia water to the filtrate, produces a precipitate of hydrastine that is much purer than the first precipitate; providing the operator had taken the precaution to add hydrochloric acid enough to the original percolate to separate the berberine, and had waited for it to separate before filtering the liquid from the precipitated hydrochlorate of berberine.
If, however, the hydrochlorate of berberine has been but partially thrown down, in consequence of an insufficient amount of hydrochloric acid having been added to the percolate, the second precipitate is of a deep yellow color, and may be mixed with yellow nodules of impure berberine.
If the percolate is very concentrated, the chloride of ammonium may be in sufficient amount to keep the liquid of acid reaction until nearly all of the hydrastine is precipitated.
3rd. It is not safe to argue that because a distinct acid reaction (with HCl) will precipitate most of the berberine from an aqueous solution of pure berberine, and because a slight alkaline reaction is sufficient to throw down all of the hydrastine from an aqueous solution of pure hydrochlorate of hydrastine, these results will necessarily follow with an aqueous percolate of hydrastis. This liquid differs in solvent powers from pure water, and the natural combination in which these alkaloids exists is far stronger than any artificial union that we have been able to make by associating them together, after they have been purified. Indeed, we have no reason to hesitate in saying that we have failed to find satisfactory evidence to disprove the supposition that berberine and hydrastine exist in the rhizome as a double salt.
4th. If Mr. Hale's process of adding ammonia water in fractions, one to neutralization and the other to excess, produced two precipitates, why will not the immediate addition of a strong excess of ammonia throw down these two as a mixture? If such a mixture is obtained, it should contain the third alkaloid.
It has been our experience to work some thousands of pounds of hydrastis each year for the alkaloids. We obtain by this process a precipitate that contains hydrastine, berberine, and some other products, but we have not been able to purify the crystallizable yellow third alkaloid.
We have, also, time and again, followed Mr. Hale's directions, while working large amounts of the drug. We obtain a second precipitate, but by appropriate methods the yellow, bitter substance resolves itself into berberine.
Method of Separation.—There are several processes whereby this object can be accomplished, but one of the most successful is as follows:
Dry the precipitate, powder it, and then extract it with boiling alcohol and filter, which will leave the hydroxides of the alkaline earths; distil most of the alcohol and add the syrupy residue to several times its weight of water acidulated with sulphuric acid; filter and precipitate the filtrate with an excess of ammonia water; collect this precipitate, dry, and powder it. Then mix it with ten times its weight of cold alcohol and acidulate with sulphuric acid. The hydrastine dissolves, forming sulphate of hydrastine, while most of the berberine remains insoluble as sulphate of berberine. Collect and wash the precipitate, and purify it by re-crystallizations from hot alcohol. The product is sulphate of berberine. By collecting the residues, of the various steps, treating them again in the same manner, and repeating the operation, the crystallizable bitter yellow substance can be mostly separated, and will also be found to be berberine.
The hydrastine of the alcoholic solution contains still considerable berberine, which can be separated by repeated crystallizations. [We do not wish to be understood as saying that berberine is the only yellow substance present in the crude precipitate. It is not, for resinous and other bodies exist in it. To our experience, however, impure berberine is the only body that conforms to the third alkaloid.] By this method the two alkaloids can be separated from each other and from the associated impurities; the berberine crystallized as sulphate of berberine, the hydrastine as the pure alkaloid. The result of one experiment of this kind, in which the precipitated crude hydrastine from several hundred pounds of hydrastis had been well washed with water, is recorded as follows:
Ninety-six ounces of crude precipitated hydrastine yielded 3 ½ ounces of pure crystallized sulphate of berberine, and 79 ounces of crystallized hydrastine. The residues did not seem to contain any substance to conform with the third alkaloid. Thus it happens that each attempt we have made to obtain this substance has failed.
Naturally, others have been interested in the matter, and, although we have questioned manufacturers of alkaloids, none have yet to our knowledge obtained it. Prof. Edward S. Wayne informs us that he has not been successful. Mr. J. W. Forbes, who for some years worked hydrastis in considerable quantities, recently denied its existence.
In order to determine if by any oversight of manipulation we were being misled, we laid the result of our work before Prof. A. B. Prescott (Mr. A. K. Hale was in his class at the time he made his determination), and sent to Prof. Prescott a sufficient quantity of the percolate to go over the matter. His investigation did not terminate successfully, and he kindly wrote us to that effect; remarking that this alkaloid doubtless should be ranked among the substances that had been recorded without sufficient examination.
Finally, we made a lot of the crude precipitate according to Mr. Hale's process; purified it, and separated the yellow crystalline sulphate (berberine), from the white alkaloid (hydrastine); and then sent a portion of each in a perfectly pure form to Prof. F. B. Power; and the yellow sulphate to Prof. Virgil Coblentz. Neither of these gentlemen were aware of the method employed in producing them, and their combustions supported each other. The yellow substance had the composition C20H17NO4.H2SO4; the white crystals were C22H23NO6.
Having thus reviewed this subject, we can only answer Mr. Hale's query by saying that the substance obtained by his process is, in our opinion, a mixture, and that the yellow crystalline body is impure hydrochlorate of berberine. We think that Mr. Hale's error has been caused by the small amount of hydrastis used in the investigation, which we understand was less than five pounds, and we believe that, had he obtained the substance in sufficiently large quantities, he would, in purifying it, have discovered its complex nature.
Other Constituents of Hydrastis.—There are additional constituents, sonic of which are of considerable interest in a general way, but none have come into use in medicine.
A fluorescent body exists in very small amount, and adheres to the hydrastine with considerable tenacity, but is mostly separated during the last crystallizations. It is soluble in chloroform, and is more soluble than hydrastine, in cold alcohol. Its solution in cold alcohol is colorless, but with a strong blue fluorescence. If we mistake not, it has been recorded that hydrastine possesses fluorescent properties, but our experience is to a contrary effect. When crude hydrastine in considerable amount is dissolved in acidulated water, and the solution is rendered alkaline with ammonia water, sufficient of this principle remains in solution to impart a deep blue color. Since it presents fluorescent properties in alkaline solution instead of in acid liquids, it may be the same as aesculin, which substance is asserted to be identical with the fluorescent principle of Gelsemium sempervirens.
Among the products that precipitate in making berberine and hydrastine when the liquid from which the alcohol was distilled is mixed with water, are a greenish oil and acid bodies that may prove of considerable interest, as shown by Prof. E. Scheffer, who had them under consideration some time ago.
Hydrastine and berberine exist in natural combination with at least one acid, of a purely sour taste, which we obtained in considerable amount as a syrupy solution, but just as we completed its purification our laboratory and all its contents were destroyed by fire. We made it by throwing out the sulphuric acid from the refuse of sulphate of berberine, by means of carbonate of barium, and after purifying the barium salt of the vegetable acid, decomposing it with an exact amount of sulphuric acid. We shall repeat the experiment.
It is to be presumed that some of the other products of hydrastis will prove of great interest to the investigator.
Powdered Hydrastis.—The consideration of this substance would naturally follow that of the drug, but we have thought it best to first introduce the constituents of hydrastis, inasmuch as the quality of the powdered rhizome really depends upon the proportions of these substances. The history of our powdered drugs is, in many instances, not an inviting one, and hydrastis seems not to have escaped the stigma that is affixed to many other substances of this nature. It is true that, as a rule, the price of the rhizome is but a trifle, and yet it may perhaps be safely said that where there is a desire to cheapen a drug, it matters little how cheap it may be, something can be found to mix with it that is less expensive. However, we do not accept that an inferior powder must necessarily be deficient in quality from an intentional adulteration. The remarks we have made in the preceding pages, regarding the variation in quality of crude hydrastis will indicate that the powder may really be from the rhizome of hydrastis; unmixed with extraneous substances and still be of inferior quality. If a worthless drug is employed the powder can not be an improvement on the crude material. It is true, we think, that the inferior qualities of many American drugs may find their way into commercial powders. This, doubtless, was true to a greater extent formerly than at present. In our opinion wholesale druggists generally desire to furnish the better qualities of all drugs, crude or powdered. That they can not always do so is perhaps largely because it is understood too often that the value of a given drug is the same, regardless of its quality; and none will deny the strong competition that the price brings to bear on dealers. Pharmacists are, in our opinion, more careful than formerly, and by the united efforts of these two bodies of men, pharmacists and jobbers, we doubt not that the progress towards a better day will continue. If we are correct, the present day is far in advance of a few years ago. We doubt if the time has ever been in the history of this country (since pharmacists commenced depending on dealers for their powdered drugs), that the qualities were equal to those of the present. The causes for an inferior powdered hydrastis, aside from intentional admixture, are the same as for the inferior drug. In considering the powdered hydrastis of commerce, should we, therefore, compare it with the average quality of the crude material such as is accepted without objection by a good pharmacist, or, with the choicest that can be obtained?
We must now leave this matter with the reader to judge as to the attention that is given this subject by pharmacists at large; but it seems to us that a dealer in a substance like powdered hydrastis can not be very severely criticised for supplying an inferior powder (shown to be inferior by analysis), if the same quality of crude hydrastis is accepted without objection by those who should act as authorities. It would be out of place for us to argue the question here, as to whether the standard of powdered hydrastis should be higher than that of the drug, but this phase of the question can with propriety be applied to other American drugs. It is a subject that will confront us before many years.
The description of hydrastis, as given in the United States Pharmacopoeia, is not such as can afford a standard of comparison. There is no recognition of the powdered drug in that work, and no standard for the crude other than that derived from a description of the physical appearances.
In 1882, Mr. C. B. Allaire presented a report to the American Pharmaceutical Association, in which he records the microscopic examination of eleven specimens of commercial powdered hydrastis, all of which were adulterated. Mr. Allaire informs us, in a communication, that many of these specimens had been intentionally mixed with extraneous substances, but that in some instances the admixture was an earth that might have been present in the unwashed drug. However, it constituted such a large percentage that it could only be viewed in the light of an adulterant.
In 1883, Mr. E. C. Bassett, then in the chemical laboratory of the University of Michigan, examined, by the microscope, eighteen specimens of commercial powdered hydrastis. Of these, twelve were unadulterated; three contained a little curcuma as a coloring; one was about one fifth curcuma; one a mixture of curcuma and bean starch; and one curcuma and a foreign root that could not be identified. Thus it appears that two-thirds of these specimens were free from admixtures.
However, while the microscope will detect such foreign substances as may be mechanically added to the powdered drug, or powdered with it, it is obvious that it can not indicate the comparative value of the specimens that are unmixed; and it is essential that a chemical method of detection be employed under such circumstances. That it can be made readily and simply is demonstrated by the nature of the constituents, and as at present the berberine is considered the important one, a comparison of the proportions of berberine is probably our best method of standardizing the drug. [Since this sentence was in type, the investigations of eminent medical authorities have drawn attention particularly to Hydrastine, and the indications are that this alkaloid may become the most important constituent.]
Appearance of Powdered Hydrastis.—This powder is not a bright yellow. Upon the contrary, it is usually of a dull yellowish hue, and often with a slight tinge of green. The brown surface of the rhizome and rootlets, and the decayed fragments that are always more or less intermixed with the crude drug, destroying the rich yellow that would otherwise be a characteristic; and thus, if commercial powdered hydrastis, is a bright yellow, it is perhaps open to suspicion. Powdered hydrastis has the characteristic odor of the rhizome, as described on page 85 of this publication.
Estimation of Berberine in Powdered Hydrastis.—The remarks that we have made in the preceding pages on the berberine subject will indicate that a method of estimating this alkaloid under one condition may perhaps be unreliable under certain other circumstances.
We shall not consume time in this place with the difficulties that accompany the processes that we have tried; for in the future we must consider this alkaloid in a broader field than it occupies in this one plant, and our remarks will then be more pertinent. The fact that it is associated with one, and perhaps, other alkaloids, necessitates a scheme that will disentangle it from such associations or combinations, and it is desirable also that the scheme should be as simple as possible and as easily applied as is practicable. We prefer the following process: [The berberine is not as completely extracted by this as by a process that we shall introduce at a future day; but for simplicity this process is desirable.]
Reduce the hydrastis to an impalpable powder, if it is not already in that condition, and then macerate one part of the powder with eleven parts of officinal alcohol, shaking often. After four days permit the powder to subside completely, and decant the overlying liquid. Add to the magma sufficient alcohol to produce the original bulk, and repeat the operation. Repeat the maceration with a third portion of alcohol and decant as before. Mix these decanted liquids, and after twelve hours filter them, washing the filter paper with a little alcohol. Add to the filtrate one-third its bulk of officinal sulphuric ether, and then hydrochloric acid to the extent of three-tenths, and sulphuric acid to the extent of one-tenth the weight of the hydrastis employed. Place the liquid, after mixing well, in a cool place, and after forty-eight hours collect the crystalline precipitate on a filter paper and wash it with a mixture of equal parts of sulphuric ether and alcohol until the crystals are free from uncombined acid; then dry it at a temperature of 125° Fah. and weigh it.
This process practically abstracts from the hydrastis its berberine, and precipitates it almost completely and as a nearly pure salt. It is true that some may prefer to employ percolation, but to our experience, in unskillful hands the process of maceration is less likely to be followed by variation in product. We do not deny that some berberine remains in the drug, for by another process the extraction is more perfect; but this process will answer as a method of comparison.
The addition of the sulphuric ether to the alcoholic solution produces a menstruum in which, if acidulated as we direct, the hydrochlorate of berberine is so nearly insoluble as to leave no trace of bitterness after separation of the salt. [Hydrastis contains coloring matters besides berberine, hence the liquid is not decolorized.] It must be also observed that, while this process is capable of precipitating a larger amount of berberine than can be obtained by the process we use in making hydrochlorate of berberine (see p. 113), it is less economical on a manufacturing scale. for the increased yield is more than counterbalanced by the expense of the ethereal menstruum; and at the usual price of hydrastis it is false economy to carry the extraction of the drug beyond a limit that is sufficient to repay in yield of berberine, the loss of material and the time consumed. Hence, in connection with our remarks on page 137, in which we present the average economical yield of berberine from ordinary commercial hydrastis, we record the following table, which gives us the comparative qualities of powdered hydrastis, as found in the American market: [This line of experiment was instituted by Mr. Leslie Soule in our laboratory, the method of investigation being in accordance with the scheme announced on preceding page. The specimens came from Indianapolis, Philadelphia, Little Rock, Louisville, Zanesville, South Bend, Ind., Pottsville, Pa., Chillicothe, O., and Lynn, Mass. Equal amounts of each were operated upon, and all carried simultaneously until the work was completed.]
TABLE SHOWING THE EXAMINATION OF FORTY-NINE SPECIMENS OF COMMERCIAL POWDERED HYDRASTIS.
| Specimen | 1 yielded from 60 parts powdered Hydrastis | 1.34 parts Berberine Hydrochlorate, equalling | 2.23 per cent. |
| 2 | 1.335 | 2.22 | |
| 3 | 1.26 | 2.10 | |
| 4 | 1.25 | 2.08 | |
| 5 | 1.25 | 2.08 | |
| 6 | 123 | 2.05 | |
| 7 | 1.21 | 2.04 | |
| 8 | 1.19 | 1.98 | |
| 9 | 1.19 | 1.98 | |
| 10 | 1.18 | 1.96 | |
| 11 | 1.16 | 1.93 | |
| 12 | 1.15 | 1.91 | |
| 13 | 1.12 | 1.86 | |
| 14 | 1.12 | 1.86 | |
| 15 | 1.10 | 1.83 | |
| 16 | 1.10 | 1.83 | |
| 17 | 1,10 | 1.83 | |
| 18 | 1.08 | 1.80 | |
| 19 | 1.08 | 1.80 | |
| 20 | 1.08 | 1.80 | |
| 21 | 1.08 | 1.80 | |
| 23 | 1.08 | 1.80 | |
| 23 | 1.05 | 1.75 | |
| 24 | 1.04 | 3.73 | |
| 25 | 1.04 | 1.73 | |
| 26 | 1.01 | 1.68 | |
| Specimen | 27 yielded from 60 parts Powdered Hydrastis | 0.98 parts Berberine Hydrochlorate, equalling | 1.63 per cent |
| 28 | 0.97 | 1.61 | |
| 29 | 0.97 | 1.61 | |
| 30 | 0.97 | 1.61 | |
| 31 | 0.94 | 1.56 | |
| 32 | 0.94 | 1.56 | |
| 33 | 0.91 | 1.51 | |
| 34 | 0.90 | 1.50 | |
| 35 | 0.85 | 1.49 | |
| 36 | 0.83 | 1.37 | |
| 37 | 0.83 | 1.37 | |
| 38 | 0.79 | 1.31 | |
| 39 | 0.70 | 1.16 | |
| 40 | 0.70 | 1.16 | |
| 41 | 0.62 | 1.03 | |
| 42 | 0.61 | 1.01 | |
| 43 | 0.57 | 0.95 | |
| 44 | 0.54 | 0.90 | |
| 45 | 0.35 | 0.58 | |
| 46 | 0.31 | 0.51 | |
| 47 | 0.24 | 0.40 | |
| 48 | 0.21 | 0.35 | |
| 49 | 0.205 | 0.34
REMARKS.—It will be observed that the yield of hydrochlorate of berberine varies from 2.23 per cent. to 0.34 per cent. Twenty-seven of the specimens were below the average working yield (1.8 per cent.) of fresh commercial hydrastis, as recorded on page 137, and seventeen specimens were above it. Five of the specimens gave the exact amount. It may safely be said that the specimens below this were inferior, for a quality of hydrastis that yields 1.8 per cent. by our working process will assay considerably better; and our experience is that the average assay of berberine hydrochlorate is not less than 2 per cent. That it may be above this is shown by the first seven specimens of our table. Averaging, however, those recorded above 1.8 per cent., we have a result of 1.98 ½. The powdered hydrastis of commerce should, in our opinion, not only reach this figure by this process, but assay 2 per cent. Allowing, however, for age and imperfect rhizomes, which some may contend should have a consideration, we may possibly lower the figure to 1.95 per cent. It will be observed that of the 49 specimens assayed but 10 reached this standard and 7 were actually less than one per cent. Of these very low specimens we can only say that, even though gathered in early spring-time and imperfectly cured, we have never met with so small a yield of berberine from hydrastis, and there is but one inference in regard to the matter. The pharmacist who purchases such a powder pays an exorbitant price when quality is considered. The physician who prescribes such a drug can not hope for a positive action.
To sum up, accepting the berberine as a standard, commercial powdered hydrastis as found in the drug market of this country is nearly four-fifths below grade, and a very considerable proportion of it is certainly adulterated with foreign bodies, or it may be with the dried and powdered hydrastis muck from which the alkaloids have been extracted.
The Detection of Curcuma in Powdered Hydrastis. [Mr. E. S. Ely made in our laboratory a series of examinations of hydrastis, curcuma, and admixtures of hydrastis with curcuma and such indigenous drugs as we have found associated with hydrastis, This interesting paper is to be found in the Druggists' Circular, May, 1885, and in acknowledgment of his work, and that of Mr. Soule, we herewith extend our thanks for their value to this publication. The necessity for the introduction of the test for curcuma is evident from Mr. E. C. Bassett's investigations (see page 144).]—Solution of caustic potassa with curcuma gives an immediate deep orange brown coloration which, in the course of a few hours, assumes a decided purple hue. With pure powdered hydrastis no change occurs. In mixtures of the two the deepness of both the primal and ultimate colors is in direct proportion to the curcuma. Hydrochloric acid furnishes a somewhat lighter orange red which slowly fades to a pink with curcuma, but no coloration whatever with the pure hydrastis. In mixtures of the two the same colors are produced as with curcuma alone, but varying in density according to the percentage of the latter. The caustic potassa solution is much the more delicate test of the two, giving immediate and distinct colorations where the acid entirely failed. The best method to conduct the foregoing tests is to place about a drachm of the suspected powder upon white filtering paper, and then carefully drop sulphuric ether upon it until the coloring matter is well extracted and diffused over the surface beyond the powder. The ether is allowed to evaporate, when a drop of caustic potassa or hydrochloric acid is added to the colored portion of the paper. A coloration will follow if curcuma is present, as described under tincture of hydrastis.
This test is so delicate as to actually show an admixture of one part of curcuma with 10,000 parts of hydrastis, a perceptible delicate red color appearing at the margin of the spreading alkaline liquid as it passes through the ether stain.
Continued on next page.
Drugs and Medicines of North America, 1884-1887, was written by John Uri Lloyd and Curtis G. Lloyd.

