Hydrastis: Adulterations - Constituents.
Continued from previous page.
Adulterations - Pharmacopoeial history - Constituents
ADULTERATIONS.—The substances which usually contaminate our indigenous drugs, are to be found mixed with hydrastis. Fragments of foreign roots, such as serpentaria, cypripedium, senega, collinsonia, jeffersonia, trillium, [We once mentioned this fact, using the name beth root instead of trillium. As a consequence, we found it copied into journals on each side of the Atlantic as beet root, a substance that could not well be used as an admixture with hydrastis.] etc., are common, and these admixtures usually result from carelessness of the collector. In a few (exceptional) cases, however, we have found them to constitute more than half the gross weight of several bales of the drug; and under these circumstances the admixtures were intentional.
 The root of Stylophorum diphyllum [This plant is of interest, and will be considered by us in our publication in its proper place.] resembles hydrastis in color when fresh, having a golden yellow juice, but it changes throughout to a dirty gray upon drying. Our attention was once called to a lot of one hundred pounds entirely made up of the root of this plant, which was thrown upon the market as an extra "Large Golden Seal." The appearance of this root will not permit of a confusion of it with the rhizome of hydrastis. (See Fig. 30). In our opinion, the color and peculiar odor of dried hydrastis will prevent any careful person from mistaking it for the root of any other plant known to us. However, very much inferior hydrastis is in commerce, some of it objectionable because of its having been gathered too early in the season, other portions because of dirt, mould, or admixtures. In consequence of these facts, much of it is unfit for use, and purchasers should exercise care in its selection.
The root of Stylophorum diphyllum [This plant is of interest, and will be considered by us in our publication in its proper place.] resembles hydrastis in color when fresh, having a golden yellow juice, but it changes throughout to a dirty gray upon drying. Our attention was once called to a lot of one hundred pounds entirely made up of the root of this plant, which was thrown upon the market as an extra "Large Golden Seal." The appearance of this root will not permit of a confusion of it with the rhizome of hydrastis. (See Fig. 30). In our opinion, the color and peculiar odor of dried hydrastis will prevent any careful person from mistaking it for the root of any other plant known to us. However, very much inferior hydrastis is in commerce, some of it objectionable because of its having been gathered too early in the season, other portions because of dirt, mould, or admixtures. In consequence of these facts, much of it is unfit for use, and purchasers should exercise care in its selection.
PHARMACOPOEIAL HISTORY.—Hydrastis had never been recognized by any Pharmacopoeia until it was made officinal in the Pharmacopoeia of the United States, in 1860, as "the root of Hydrastis canadensis," and then no preparation of it was introduced.
The Pharmacopoeia of 1870 continued it under the same name, and authorized the preparation of a fluid extract of hydrastis.
The revision of 1880 recognized it as "the rhizome and rootlets of Hydrastis canadensis." This revision continued the fluid extract, and introduced a tincture.
CONSTITUENTS.—Berberine: History of the Name of this Alkaloid.—In 1824, Huttenschmid discovered a substance in the bark of Geffroya inermis, and gave it the name jamaicine. This Wittstein (Organic Principles of Plants, p. 26) accepts as berberine. [Gmelin overlooked the work of Huttenschmid, and ascribed, in his Hand-Book of Chemistry, the discovery of berberine to Chevallier and Pelletan. Compare also the statements of J. Dyson Perrins, in the Journal of the Chemical Society (1863), and its reprint in the Pharmaceutical Journal and Transactions (1863), p. 464.]
Chevallier and Pelletan discovered it in the bark of Xanthoxylum Clava Herculis (1826), and named it xanthopicrite, a name that could have been very appropriately applied to this rich, yellow alkaloid.
Rafinesque (1828) named the yellow coloring matter of Hydrastis canadensis hydrastine. [Medical Flora of the United States, 1828, Vol. I., p 253.]
Buchner and Herberger (1830) gave the name berberine to a purified extract of Berberis vulgaris, although Brandes previously (1825) may be said to have described a yellow coloring matter that he obtained from this plant. [American Journal of Pharmacy, Vol. III., 1831, p. 173. Also Gmelin's Hand-Book of Chemistry, Vol. XVII., p. 186.] He did not ascribe a name to it.
Thus it will be seen, accepting all of these substances to be identical, that the name berberine appeared last.
In reviewing the record, we are at a loss to determine why the names that were entitled to the precedence should have been displaced by the term berberine. It may be argued that the words jamaicine and xanthopicrite were not affixed to definite proximate principles, but since the name berberine was originally applied to a solid extract, we can not argue in its favor from that view. The word hydrastine, announced by Rafinesque in 1828, was overlooked by all writers, so that this term could not have entered the lists even had it been known at an early day that this substance was identical with berberine. Therefore the name least entitled to the honor from a chronological standpoint is the term berberine, which by common consent has been accepted.
History of the Alkaloid Berberine.—Authorities have recorded the history of this alkaloid in Europe. Since they overlooked the American history, or were not conversant with it, we shall introduce it, and in connection endeavor to review the entire matter, which can not but be of general interest.
As before stated, Huttenschmid (1824) gave us the name jamaicine; Chevallier and Pelletan (1826) gave us the name xanthopicrite; Rafinesque (1828) introduced the name hydrastine; and finally (1830) Buchner and Herberger announced berberine.
The fact that Rafinesque had entered the lists seems to have then been unknown, and we can find no recognition of him by subsequent investigators; and it seems to us an oversight in passing the work of this eccentric but talented scientist. [It is true that Rafinesque took the broadest liberties with the sciences in which he wrote, and few will deny that he was very egotistical. However, he was a persistent student, and his works become more valued as the years pass. He entered the field as a writer in several branches of the Natural Science of his day, and it is now recognized that many of those works are among the most difficult to obtain. His "Medical Flora" is rare indeed, and his work upon Fishes is entirely out of market.] His "Medical Flora of the United States" (Vol. I.) was written between the years 1816 and 1828, being published at the latter date. In it (p. 253) he defined the yellow alkaloid of Hydrastis canadensis as "a peculiar principle hydrastine, of a yellow color." We fail to find a better description of this alkaloid until years afterward, for Rafinesque individualized hydrastine, and pointed to it as the prominent principle, by saying of hydrastis, "It contains amarine, extractive, several salts, and a peculiar principle Hydrastine [Italicized by Rafinesque.] of a yellow color."
We are thus careful in giving this record because the name hydrastine was accepted by a very considerable body of practitioners (Eclectic), and in American commerce it is now hydrastine. [When it was shown by Mahla, 1862, that the substance employed by Eclectics was identical with berberine, they (Eclectics) would more readily have accepted the name berberine if it had quietly been announced. Considerable feeling once existed relative to this substance, and Eclectics were not willing to be driven into the use of a name that from their view came after the name hydrastine. But that feeling has passed, and the least said the better.]
In continuing the history, we find that in 1830, when Buchner and Herberger announced the name berberine, it was applied to a purified extract of Berberis vulgaris. [Compare Am. Journ. Pharm., 1836, p. 368, from Journ. de Pharm., 1835.] The substance obtained by them was neither berberine nor a salt of berberine, and the berberine present in their extract could have only represented a small portion of the product. Their process will not admit of any other view of the subject, and the yield of berberine they report, seventeen per cent., can not be obtained from Berberis vulgaris. Hence the name berberine was not originally applied to an alkaloid.
In 1835, Prof. Buchner and son obtained, unknowingly, the hydrochlorate of berberine in crystalline form, but thought it a neutral principal, or a weak vegetable acid, and thus we may ascribe to the Messrs. Buchner the honor of really obtaining from Berberis vulgaris the first salt of berberine. [The date of its appearance is variously stated at 1830, 1832 and 1835. Dr. F. F. Mayer refers to Buchner's Report. xxxvi., p. 1, 1830, for the original paper (not at our command). See also Pharmaceutical Journal and Transactions, 1863, p. 517. We therefore accept 1830 as being well authenticated.]
Dr. George Kemp, in 1839, assigned berberine to a place among the alkaloids, producing a combination with picric acid. He recorded his experiments in Buchner's Reportorium, 184o; but this fact seems to have been overlooked. In 1841 he investigated the substance more thoroughly, producing a hydrochlorate, sulphate, acetate, and some other salts; but in consideration of a request from his friend Prof. Buchner, who wished his (Buchner's) son to reexamine the subject, Kemp withheld his paper from publication. [Chemical Gazette, 1847, p. 209.]
Thus it occurred, that in 1847, Thomas Fleitman, unconscious of Kemp's work, published an essay on berberine and its salts, without recognition of Dr. Kemp's labors in the same field. He demonstrated that berberine was neither a neutral coloring matter, nor a weak acid, as the Messrs. Buchner had supposed, but a true alkaloid, and strongly basic. He even examined a portion of the substance made by the Messrs. Buchner in 1835, and supposed by them to be either a neutral principle or a vegetable acid, and found it to be hydrochlorate of berberine. And outside of Mr. Fleitman's report, the testimony from the Messrs. Buchner's description is, in our opinion, to the effect that they obtained hydrochlorate of berberine. They described their product as "A very light powder, composed of acicular crystals, of a bright lemon yellow color, very slightly soluble in cold water." This description will not apply to the alkaloid.
Hence we find that Thomas Fleitman gave to the world (1847) the first general intimation of the basic character of berberine, and he is, therefore, accepted by most writers as having assigned it to a place among the alkaloids, although it is established that it had been known by Dr. Kemp to be an alkaloid from the year 1839. [Mr. Fleitman's paper may be found in the Chemical Gazette, 1847, p. 129, and following it in a subsequent number, p. 209, the statement from Dr. Kemp, that he (Kemp) had long known of the basic character of berberine. and had remained silent out of respect to the request of Prof. Buchner.]
History of the Yellow Alkaloid (Berberine), as Obtained from Hydrastis Canadensis (1828), Originally Called Hydrastine.—We will again repeat, that in America the name hydrastine was originally given, by Prof. Rafinesque, to this alkaloid, which is the principal coloring matter of Hydrastis canadensis, and that he gave it before the name berberine appeared in Europe. By this name it was accepted when introduced into American medicine by the Eclectics (1847), Rafinesque's works being prominently recognized by this section of the medical fraternity. In our botanical history of Hydrastis (p. 83), we presume to regret that the appropriate name of Ellis (Warneria), was not continued to the plant, instead of the illogical name Hydrastis. We also think it unfortunate that, since the name Hydrastis was accepted by botanists, it was not followed by chemists in the naming of its prominent constituent, the yellow alkaloid.
No printed process for making the yellow alkaloid (berberine) appeared before 1851, and we must consider that Mr. Durand first announced a salt of berberine from Hydrastis canadensis, although he was unconscious that it was a salt. In the year 1850, he wrote a paper on Hydrastis, and published it in the American Journal of Pharmacy, April, 1851, in which he called attention to "a yellow coloring matter" made by precipitating an alcoholic tincture of the root of Hydrastis canadensis by means of a solution of bichloride of tin, describing it as "a most brilliant yellow precipitate." This substance Mr. Durand neglected to investigate, but suggested that it might "prove a useful pigment in oil and water painting." It was hydrochlorate of berberine, and thus he was the first to obtain a salt of this alkaloid from Hydrastis canadensis and record the fact. [By a coincidence, the first preparation of the alkaloid used in American medicine was also considered a neutral principle, and in reality was hydrochlorate of berberine. It was called Hydrastine Neutral, being made from Hydrastis canadensis.] At this time Eclectic physicians were using the substance as a remedy under the name Hydrastine, or Neutral Hydrastine, and hence it is that the hydrochlorate of the alkaloid was the first definite preparation supplied to the medical profession of America. [At first sight it may seem strange that the investigators, without exception, failed to ascribe to this substance alkaloidal properties. It was discovered independently in different plants, by several persons, as our history will show, and in no instance was it identified. Upon deliberation, however, at will be seen that in that early day the alkaloidal tests now so easily applied were unknown. Therefore an alkaloid. forming with hydrochloric acid an almost insoluble salt, was an exception to all known alkaloids, and consequently not likely to be compared with other organic basis.]
Although Mr. Durand prepared the hydrochlorate of the alkaloid berberine, in 1850, from Hydrastis, and Pharmacists who made Eclectic medicines had supplied it to the medical profession in considerable quantities from before that period, neither had identified it as berberine, or as a salt of that alkaloid, although it was certainly known, by a few, to possess alkaloidal properties. [See Grover Coe's work, positive Medicinal Agents, 1855. And we refer the reader to our historical introduction of hydrochlorate of berberine for some points in this connection.]
Nothing appeard after Mr. Durand's work for a period of twelve years; but in 1862 the subject was taken up by Mr. F. Mahla, of Chicago, and in a paper contributed to the American Journal of Science and Arts, January, 1862, he clearly established the fact, that the Eclectic "Hydrastine" was the salt of an alkaloid, and that this was berberine. Therefore, to Mr. Mahla is due the credit of really identifying as berberine the alkaloid that had been discovered fifteen years previously in Hydrastis canadensis. [We thus see that the American history coincides remarkably with the European, for in 1824 Huttenschmid discovered berberine, and fifteen years afterward Kemp identified it as an alkaloid, although between those periods it had been discovered independently, and examined by several good authorities.] In this connection we must not forget to record the fact, that Mr. J. Dyson Perrins, of England, really discovered berberine in Hydrastis before Mr. Mahla identified it, but he neglected to announce the fact. He states in the Journal of the Chemical Society, 1863, that "sometime before the publication of Mahla's paper, I had noticed the occurrence of berberine in Hydrastis canadensis," and thus Mr. Perrins stands in comparatively the same position as Dr. Kemp, both having anticipated the work of the persons who made the announcements.
The Past and the Future Name of this Alkaloid.—It may seem that we overstep the line of prudence, and pass into a field that we should not presume to enter, when we even announce a heading such as the above. We trust, however, that our experience with this almost exclusively American drug, our aggravations commercially, and our endeavor to familiarize ourselves with its past record, will excuse us to the reader, if we cautiously consider the future.
There can be no doubt that the name berberine is applied to the alkaloid by a comparatively small number of American pharmacists and physicians, and that in America the recognized name is still Rafinesque's "Hydrastine." The endeavor to affix the term berberine to this yellow alkaloid of Hydrastis canadensis, has as yet proven a commercial failure. It is true, that with scientific men and many writers, berberine is acknowledged, but these men are few, compared with those who use the term hydrastine. The question that naturally presents itself is, are the men who prefer hydrastine entitled to consideration? Although we support the term berberine, we must acknowledge the justice of the name hydrastine from the following reasons:
1. The name hydrastine was applied before the name berberine, the one in America (hydrastine), the other in Europe (berberine).
2. This substance and its salts, under the name hydrastine, hydrastine muriate, etc., came into extensive use in America, and so generally, that at the present day we estimate that from 25,000 to 28,000 pounds of Hydrastis canadensis are annually consumed in making the alkaloid and its salts. They are scarcely used in Europe.
3. This name (hydrastine) has become so strongly fixed in the trade interests of our country, that for this reason alone we would even now acknowledge its claims for primary recognition, were our country only to be considered.
However, even though the name hydrastine is chronologically entitled to preference, and though the amount of the alkaloid produced from Hydrastis canadensis for medicinal use, in America, is doubtless very much greater than that from all other sources the world over, we think that the fact of its being familiar to scientists of all countries as berberine, now entitles that word to preference.
Throughout America the name hydrastine is as firmly engrafted as before Mahla (1862) announced that hydrastine and berberine were identical. There is little indication that the term hydrastine will be supplanted by berberine at any immediate day, yet in common with others we have always given our assistance towards bringing about this result. All have failed, and the public seems to tenaciously insist that commercial precedence, and the source of the drug, shall have precedence in the recognition of a name. Hence, in America the name berberine is applied by a few, and hydrastine by the many.
It is not unlikely, however, that if the leaders in the various schools of medicine and in pharmacy will endeavor to bring about uniformity in expression, and will use the word berberine whenever it is possible, it can be made the name of the future. [Few realize the hold of the word hydrastine in America. When we consider that it is applied to a proximate principle that has been used extensively for twenty years, and that the name gives the origin of the drug, we can appreciate the fact that it will be displaced very slowly, There is another argument against the word berberine, and that is the resemblance to beeberine. These substances are often confused in commerce, and confounded by physicians, and that they so nearly resemble is unfortunate.]
Processes Announced for the Preparation of Berberine.—It would be natural to suppose that a substance of the importance of berberine, and studied as this substance has been during a number of years, could now be readily prepared in a state of purity. We will venture to say, however, that according to our investigations, the production of this alkaloid, free from contaminations and decomposition products, is by no means an easy matter. A personal experience of some years on a manufacturing scale by means of the formulas suggested, and accepted by many authors as reliable, has not been at all satisfactory. It is therefore necessary for us to review the processes that have been named; and while we dislike to differ, even in the least, with such excellent authorities as have considered this subject, we must not neglect to add any light that may have been cast in this direction by our work. We find, also, that others have not been altogether satisfied; and investigators who are no less conspicuous in the literature of berberine than Mr. Perrins and Prof. Wm. Procter, have doubted the constitution of the substances produced as berberine. Thus Mr. Perrins states that "the pure alkaloid itself is equally unsuited for analysis.... Indeed, I find it not easily prepared in a state of purity." And that Mr. Perrins was uncertain of the substance known to others as berberine, is evidenced by the fact that his ultimate analyses were all made of the salts of berberine. Prof. Procter, in referring to this subject, has written: [American Journal of Pharmacy, 1864, p. 10.]
"Having occasion recently for information relative to the production of pure berberine in an uncombined state, a reference to all the authorities at my disposal, including nearly all the papers published within the last few years, I noticed with some surprise that these writers, in describing berberine, treated the substance obtained from Berberis vulgaris by the agency of neutral solvents, and which, as berberine is an alkaloid, must be a neutral salt of that alkaloid."
The substance originally called berberine having been found a mixture of berberine and extractive matters, led to the suggestion of several processes for freeing this alkaloid from its combinations. Mr. Fleitmann announced the following: [Chemical Gazette, 1847, p. 129.] "Sulphate of berberine was made by decomposing the muriate with weak sulphuric acid; the salt then recrystallized, and dried at 212° F to expel all traces of muriatic acid. Baryta water was added to the solution until it became alkaline, when the liquid immediately assumed a dark red color. To remove the excess of baryta, carbonic acid was passed through the liquid, which was then boiled and filtered, upon which the dark red solution was evaporated nearly to dryness in the water bath, and dissolved in ordinary alcohol; the berberine was precipitated by ether, and recrystallized from water."
This is the process now adopted by most of the authorities we have consulted, but we regard the product as uncertain and by no means of uniform composition. He first directs the preparation of muriate of berberine, and this salt is then to be dried at a temperature of 212° F. This preliminary step introduces a possible impurity, for we are convinced that such a temperature can not be applied to the moist salts of berberine without risk of partially dissociating them. In this view we find that our experiments have also corroborated those of Mr. Perrins, who writes of muriate of berberine as follows: "I acquiesced in Fleitmann's formula, and even supposed that it was confirmed by my analysis of the hydrochlorate and by a platinum determination; but later experience has shown me that the hydrochlorate is not suited for ultimate analysis, as by pretty long exposure to a temperature of 100° C., or thereabouts, it undergoes some decomposition."
Next, we find that the addition of solution of caustic baryta until an alkaline reaction results, is a procedure that should be avoided if possible, and by no means should the alkali be added in great excess, for the equilibrium of this delicate alkaloid is likely to be disturbed by contact with excess of an alkali. Mr. J. Stenhouse noticed this dissociating power of the alkalies on berberine, and in the Journal of the Chemical Society, London, 1867, p. 187, he cautions us against their use, and considers caustic lime preferable to any of them as being less destructive.
Finally, the evaporation of the solution of berberine, after precipitation of excess of barium by a current of carbon dioxide, should not, in our opinion, be carried on at the temperature of a water bath, and most certainly not as Mr. Fleitmann directs, "nearly to dryness." Such an application of heat, especially when continued in this manner, will decompose portions of the alkaloid.
Taking these factors together—and we doubt if many workers with this alkaloid will dissent concerning their several influences—we can not but accept that the product must be uncertain. Hence, while Mr. Fleitmann obtained a body which he found to possess certain characteristics, we are not surprised that others who have followed, and excellent authorities, differ both from him and from each other.
In 1862, Mr. Wm. S. Merrell stated that berberine might be prepared by decomposing sulphate of berberine by means of oxide of lead. [American Journal of Pharmacy, 1862, p. 503.] Acting on his suggestion, Prof. Wm. Procter elaborated a formula as follows: [American Journal of Pharmacy, 1864, p. 10.] Freshly precipitated oxide of lead, basic hydroxide, Pb2O(OH)2, was digested in excess with sulphate of berberine, which had been previously dissolved in boiling water, until a filtered portion of the solution failed to strike a precipitate with solution of acetate of lead or with baryta water. It was then filtered, evaporated and crystallized.
This process seems certainly to be free from some of the objectionable features of those that have preceded, and yet (admitting that berberine can be produced) as a necessity there must be a long-continued application of heat; and this should be avoided.
Again, in our hands the process has been a complete failure in other respects, because it abstracts only a part of the sulphuric acid; and in support of our view we give a synopsis of the following experiments that we have repeatedly made.
One part (480 grains) of nitrate of lead was dissolved in water and precipitated with excess of ammonia water. The basic hydroxide so produced was well washed, and added to a solution of one part (480 grains) of berberine bisulphate (C20H17NO4H2SO4), in 32 parts of water. The mixture was digested at a temperature of 166° F. for forty-eight hours, with frequent stirring, the evaporated water being replaced, and was occasionally tested with solution of acetate of lead. [Prof. Procter states that solution of caustic baryta can also be used to determine the absence of sulphate of berberine. We believe that the lead sulphate dissolves to a considerable extent in this solution of berberine; and hence we scarcely think that the barium test is reliable.] The sulphuric acid was not withdrawn, the solution giving every evidence of being still a solution that contained a sulphate of berberine. If the liquid be evaporated to dryness, decomposition results; and upon re-solution a deep red liquid is produced, which still contains a sulphate of berberine after the lead is precipitated by means of sulphide of hydrogen. In this case, however, the sulphate conforms to the properties of the normal salt (C20H17NO4)2 H2SO4.
Under the same circumstances lead monoxide, PbO, fails to withdraw the sulphuric acid from the bisulphate of berberine. We are convinced that Prof. Procter really obtained the soluble normal sulphate as we did with our ammonia process, a compound that at that time was unknown. In this connection, we remember that Prof. Edward S. Wayne once informed us that in his hands the process was a failure.
In 1867, Mr. G. Stenhouse published in the Journal of the Chemical Society, London, a process in substance as follows: [Journal of the Chemical Society, London, 1867, p. 187.]
"One part of acetate of lead is dissolved in three parts of water, and to the boiling solution one part of very finely ground litharge is added in small portions, and heated until the whole forms a thick, pasty mass. This is then diluted with one hundred parts of water, and twenty parts of the finely ground wood is mixed with it and boiled about three hours, and strained. A little litharge is then added to the liquid, and it is evaporated to crystallization, when, 'on cooling, berberine crystallizes out in dark brown tufts of needles.'
"In order to purify the crude berberine obtained by the foregoing process, it is dissolved in boiling water, and subacetate of lead added as long as any precipitate is produced This solution, filtered while hot, almost solidifies on cooling to a mass of yellow needles, which, however, still contain lead and organic impurities. They are collected on a cloth filter, pressed, dissolved in boiling water, and sulphuretted hydrogen is passed through it. The hot solution, after filtration to separate the precipitated sulphide of lead which carries down some organic impurities, is acidulated with acetic acid and allowed to cool. The bright yellow needles of nearly pure berberine are collected, pressed, and dried at a gentle heat."
This process will not produce berberine, but an acetate of berberine. Even if the treatment with solution of basic acetate of lead yielded berberine, it would be impossible to finish the product by acidulating the solution with acetic acid, as Mr. Stenhouse directs, and avoid the formation of acetate of berberine, which is in reality the substance produced by the process. Hence those who employed this formula can not well agree in their description of the product with persons who used the process of Mr. Fleitmann.
Dr. T. L. A. Greve, of Cincinnati, suggested a process in the Eclectic Medical Journal, 1877, [Eclectic Medical Journal, Cincinnati, 1877, p. 312.] whereby muriate of berberine is decomposed by means of oxide of silver. This process certainly produces chloride of silver, with the separation of the chlorine from the alkaloidal salt, and the formation of a substance that dissolves with a deep red color, and which forms salts with acids.
Dr. Greve's plan is to make a boiling solution of muriate of berberine, and add oxide of silver in amount sufficient to decompose it. The reaction we find to be rather violent if moderately large amounts are used, and is accompanied by the evolution of gas bubbles and a hissing noise, even in the small proportions of a few grains. When we consider the unstable nature of oxide of silver when in contact with organic substances, we can not but question the production of pure berberine by this process. The result in our hands seems quite conclusive that oxidation products arise from the action of this powerful oxidizer on the berberine, and that the reaction is not so simple as to be altogether explained by a double decomposition between the two substances.
Lastly, the writer suggested that berberine could be prepared as follows: [J. U. Lloyd, in Proceedings of the American Pharmaceutical Association, 1878. See also American Journal of Pharmacy, 1879, p. 11.] "Rub eight parts of sulphate of berberine in a Wedgwood mortar, cautiously adding ammonia water until in slight excess. Pour the dark liquid into thirty-two parts of boiling alcohol, and allow the mixture to stand thirty minutes; then filter. Stir into the filtrate thirty-two parts of cold sulphuric ether, and cover tightly. Surround the vessel with ice, and allow it to stand from twelve to twenty-four hours; then separate the magma of minute crystals of berberine with a muslin strainer or filtering paper, and dry by exposure to the atmosphere."
This product is in reality a sulphate of berberine of the composition (C20H17NO4)2.H2SO4. At the time the process was announced, the writer considered the presence of sulphuric acid to be due to adhering sulphate of ammonium, but subsequent investigations have demonstrated that such is not the case; and the fact was announced in the American Druggist, 1884, Sep., 166.
After reviewing the published processes that have been brought to our attention, as announced in the foregoing pages, we must admit that this berberine subject is not in a satisfactory condition, and that the contradictory reports of those who have written on the properties of the alkaloid are doubtless mostly due to the variable condition of the product.
The Preparation of Berberine.—Our experiences with the processes that have been recorded having proved so unsatisfactory, and really in accord with the work of others, we have endeavored from time to time to obtain the alkaloid in a state of unquestionable purity. The most satisfactory process, but not by any means without objections, is based on that of Mr. Fleitmann—the decomposition of sulphate of berberine by means of solution of hydroxide of barium. [If carbonate of barium would decompose sulphate of berberine completely, the action of an alkali would be obviated. However, it will not do so.] With the precautions that we suggest, a moderate proportion of a substance can be obtained that conforms to our description of berberine, and which we believe can be accepted as the pure alkaloid.
Make a saturated solution of sulphate of berberine (C20H17NO4)2.H2SO4 [The salt used by Fleitmann and others has been the bisulphate of berberine C20H17NO4H2SO4. This is so nearly insoluble as to require heat. In order to evaporate the product, heat also is necessary in consequence of its dilute condition. We overcome this by making a cold, concentrated solution of the soluble sulphate.] in distilled water, and at a temperature of 15.5° C. cautiously add solution of hydroxide of barium until in very slight excess. Pass a current of carbon dioxide at once through the product, until it ceases to afford a precipitate with a filtered portion of the liquid, and then filter it. Place this dark red solution of berberine in a shallow vessel, and expose it to dry air under a bell glass containing a vessel of sulphuric acid, chloride of calcium, or freshly burned lime. After the liquid has reached a syrupy consistence, a deep brown crust forms over its surface which is of rather uncertain composition, as it refuses to completely re-dissolve in water. However, deep garnet red, needle-like crystals form beneath it of considerable size, distinct and clearly defined. These bear no evidence of contamination, form salts to perfection, and in our opinion are pure berberine.
This process, it will be seen, presents the following advantages over others:
- 1st. A very soluble sulphate of berberine is employed, which enables us to obtain a cold, concentrated liquid.
- 2nd. By close attention the sulphuric acid can be all withdrawn with only a slight excess of caustic baryta, which must be immediately decomposed by means of a current of carbon dioxide.
- 3d. The final evaporation is without heat; and thus from the beginning to the close of the operation the temperature need not rise above 15.5° C.
Working with very small amounts has not been satisfactory. We prefer to employ not less than a pound of sulphate.
Identity of the Alkaloid (Berberine), as obtained from Hydrastis canadensis and Berberis vulgaris.—The differences in the description of berberine has led some persons to question the identity of the substance as derived from different sources. While it is true that processes that will separate certain salts of berberine from some plants, fail to do so with others, we are convinced that this is in consequence of the natural combination of the alkaloid or the influence of associated bodies. In our hands, after purification, the alkaloid known as berberine is identical in properties, whatever has been its origin. The sulphate, muriate, and other salts of berberine as obtained by us from Hydrastis, conform in character with the same substances made from Berberis vulgaris.
In the year 1862, Dr. F. Mahla, of Chicago, presented a paper [American Journal of Science and Arts, Jan., 1862.] on the substance used by Eclectic physicians under the name Hydrastine. [See our history of the yellow alkaloid from Hydrastis canadensis.] From the reaction of its salts, and also by the support of an elementary analysis, he decided that it was the well known alkaloid berberine. He believed himself to have been the first to enter this field, for he wrote: "An organic elementary analysis of this substance [As made from Hydrastis canadensis.] does not exist;" but at the same time Mr. Perrins, of London, England, was engaged in a similar investigation of the yellow alkaloid of Hydrastis.
Mr. Perrins, after enumerating a number of plants of different natural orders, accepted as indisputable the fact that they all contain the alkaloid berberine. He is undoubtedly our best authority on this subject. He included Hydrastis canadensis, and wrote as follows before entering into the elaborate analyses he made of the berberine salts: [Journal of the Chemical Society, London, 1862, Vol. XV., p. 343.] "It seems unnecessary to state in each case from which plant I have prepared the salt for analysis; suffice it to say that the whole of the sources now first announced are included."
Mr. Perrins did not advance his method of comparison; and although there can now be little, if any, doubt that his assertions were based on experimental proof, we feel that our paper would be less perfect were we to neglect the subject.
Therefore we forwarded specimens of perfectly pure and re-crystallized sulphate and bisulphate of berberine to Prof. F. B. Power and Prof. Virgil Coblentz, and to the latter gentleman a specimen of crystallized berberine, all from Hydrastis canadensis. These substances were analyzed by Prof. Coblentz, who, after several combustions, assigned to each alkaloid the formula C20H17NO4. Prof. Power made combustions of the bisulphate only, and also reported the empirical formula C20H17NO4. In view of these facts, we think it can be accepted beyond a doubt that the yellow alkaloid of Hydrastis Canadensis is identical with that of Berberis vulgaris, and is berberine.
The Composition of Berberine (C20H17NO4).—The history of the trials through which this alkaloid has passed must be continued to its chemistry. Indeed, it would seem strange if a substance that has gone through so many other vicissitudes should not have met with troubles in this department.
Prof. Buchner (1835) first assigned to berberine the formula C33H18NO12 (old notation).
In 1847, Thos. Fleitmann redetermined it from the analysis of berberine prepared by himself, the result being C42H18NO9 (old notation). [Chemical Gazette, 1847, p. 129.] He thought that the discrepancy between himself and Prof. Buchner probably resulted from the fact that Buchner examined an impure muriate of berberine. This paper led to a communication from Dr. George Kemp, [Chemical Gazette, 1847, p. 209.] who, taking exception to Mr. Fleitmann's calculation, argued from Fleitmann's own analysis that the formula announced could not be correct. He therefore recalculated the formula from the figures of Mr. Fleitmann, and announced that C40H17NO9 (old notation) more nearly agreed with the result of the analysis. [Gerhardt also noted the discrepancy in Mr. Fleitmann's formula, and proposed the formula C42H19NO10. As shown by Mr. Perrins afterward, Mr. Fleitmann's formula was already too high in carbon.] However, from an analysis of the double chloride of berberine and platinum, he obtained the formula C42H17NO7 (old notation).
Dr. Bödeker followed this with a communication to Liebig's Annalen, lxix., p. 37, in which, from an analysis of berberine made from Columbo, he agreed with Fleitmann that it is C42H18NO9 (old notation).
Again, Dr. Hinterberger, 1852, from an analysis of the double chloride of berberine and mercury, and Kehl & Swoboda, 1853, [Chemical Gazette, 1853, p. 70, from Ann. der Chem. & Pharm., lxxxiii., p. 339.] from an analysis of a double salt of cyanide of mercury and muriate of berberine, both agree with Fleitmann's C42H18NO9.
This was the unsettled condition of affairs when Mr. J. Dyson Perrins (1862) contributed his paper on the berberine question. [Journal of the Chemical Society, London, Vol. XV., 1862. p. 339.] After reviewing the work of others, he very modestly states that "It is not without some hesitation that I allow myself to question the conclusions of chemists of the eminence of Fleitmann, Bödeker, and others; but my own results are so accordant with each other, the number of analyses I have made, and the variety of combinations I have examined are so considerable, that I feel not only justified in proposing an alteration of the formula, but, indeed, compelled to do so." He argues against Mr. Fleitmann's formula, as others had done, from his (Fleitmann's) own analysis, and says that "the numerical results... support the formula I propose rather than his own."
Mr. Perrins then presented the combustion of many salts of berberine, and ascribed to the alkaloid the formula C40H17NO8 (old notation), which is accepted to-day; and we have C20H17NO4 of the new notation.
That this formula is correct, has been settled beyond a doubt. Prof. Ernst Schmidt, of the University of Halle, [Berichte der Deutchen Chemischen Gesellschaft, 1883, No. 15, p. 2589.] states that it is C20H17NO4, as shown from numerous analyses, made of the pure base, the sulphate, hydrochlorate and nitrate; also from an examination of the hydroberberine. And lastly, we call attention to the combustions made by Prof. F. B. Powers and Prof. Virgil Coblentz (p. 105) of the berberine made by us from Hydrastis canadensis.
Properties.—[The berberine herein described was made according to our process and recrystallized from cold water over sulphuric acid. It responded to all tests for purity, was free from sulphuric acid, soluble in both water and alcohol.] Berberine crystallizes in tufts of dark brown-red needles, figure 31 representing the appearance of these crystals when slightly magnified. The crust that forms over the surface of the liquid is of a dark brown color. If a concentrated solution of berberine in alcohol be mixed with four parts of sulphuric ether a semi-crystalline magma of an orange yellow color is deposited. Micro-crystals of this are not so clearly defined and the salt is less soluble than the pure crystallized berberine. In connection with this subject we present (next page) figures 32 and 33, micro-drawings made by Mr. William J. Huck, under the personal supervision of Prof. F. B. Power, of the Department of Pharmacy, University of Wisconsin, [Figures 32, 33, 34, 36, 37, and 38 are all the work of these gentlemen and prepared for our publication.] from specimens of berberine prepared by us in the manner we have stated. Prof. Power contributes as follows:
Berberine crystallizes in tufts of dark brown-red needles, figure 31 representing the appearance of these crystals when slightly magnified. The crust that forms over the surface of the liquid is of a dark brown color. If a concentrated solution of berberine in alcohol be mixed with four parts of sulphuric ether a semi-crystalline magma of an orange yellow color is deposited. Micro-crystals of this are not so clearly defined and the salt is less soluble than the pure crystallized berberine. In connection with this subject we present (next page) figures 32 and 33, micro-drawings made by Mr. William J. Huck, under the personal supervision of Prof. F. B. Power, of the Department of Pharmacy, University of Wisconsin, [Figures 32, 33, 34, 36, 37, and 38 are all the work of these gentlemen and prepared for our publication.] from specimens of berberine prepared by us in the manner we have stated. Prof. Power contributes as follows:
"The alkaloid was presented in two forms, as a lemon yellow precipitate (made by precipitation with ether.—Ed.) without distinct crystalline structure, or as a reddish-brown crust, with a darker and somewhat crystalline exterior. Neither of these forms, however, admitted of exact representation, and the crystalline coating upon the crust, after being detached and brought under the
microscope, showed such an imperfect aggregation that a drawing would be of little value. To obtain better crystals, both the yellow precipitate and a portion of the dark-colored crust were therefore dissolved in absolute alcohol, and the solutions allowed to evaporate directly upon an object glass.
"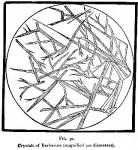 Fig. 32 represents the alkaloid berberine as precipitated from alcoholic solution by means of ether, crystallized upon an object glass, and magnified 300 diameters.
Fig. 32 represents the alkaloid berberine as precipitated from alcoholic solution by means of ether, crystallized upon an object glass, and magnified 300 diameters.
 "Fig. 33 represents the reddish-brown crust of the alkaloid berberine as crystallized upon an object glass, and magnified 300 diameters.
"Fig. 33 represents the reddish-brown crust of the alkaloid berberine as crystallized upon an object glass, and magnified 300 diameters.
"Neither of these show a definite crystalline form, while the crystals of Fig. 32 are especially irregular and much branched."
Berberine has a pure and lasting bitter taste and is odorless. It should dissolve without residue in both water and absolute alcohol.
Solubilities.—A reference to our history of the alkaloid berberine will render it unlikely that a name can be applied to so many different substances and these bodies have an accepted solubility. We find, therefore, that the solubility of berberine is stated to be all the way from one part in eighty of water, to one part in five hundred of water. According to our experience berberine varies in solubility in accordance with the proportion of the liquid to alkaloid and time of exposure, and we do not doubt that even with the same specimens different experiments will obtain discordant results. In presenting the following we will therefore say that a contact of one hundred hours was permitted in a closely-stopped bottle between the liquid and an excess of one-eighth its weight of undissolved berberine at a temperature of 15.5° C. The mixture was shaken frequently, and after perfect subsidence of undissolved matter, the clear overlying solution was decanted, weighed, evaporated at an ordinary temperature by exposure to dry air, and the residue weighed and deducted from the weight of the solution. We used one ounce of berberine in each experiment.
Forty-five and one-fifth parts by weight of the solution of berberine in anhydrous alcohol yielded 6.7 parts of dry berberine.
Thirty-three and one-seventh parts of the solution of berberine in water yielded 1.9 parts of dry berberine. Consequently, one part of berberine dissolved in 6.79 parts of absolute alcohol and in 17.77 parts of water. It readily forms super-saturated solutions with both alcohol and water.
Berberine is practically insoluble in sulphuric ether, chloroform, carbon disulphide and benzol.
Decomposition Products of Berberine.—These are not of great interest to physicians or pharmacists, but we will briefly review the important features of the work that has been done in this direction. Fleitmann found that when berberine is heated from 160° to 200° C. vapors were evolved that condense to an oily liquid, which dissolves in alcohol and is precipitated from such solution by acetate of lead and by water. Hlasiwetz heated berberine in a sealed tube with water, and obtained a substance of a red color and a bronze-like luster by transmitted light, and a green color by reflected light. He added sodium amalgam to a boiling solution of berberine and produced a hydro-berberine of basic reaction of a yellow color and the formula C20H21NO4. This formula has been confirmed (1884), by E. Schmidt, [Journal of the Chemical Society, 1884, March, p. 339.] who states that from its behavior with ethyl iodide, hydro-berberine must be a tertiary base. This author also formed berberine hydriodide by treating berberine with iodide of ethyl; and produced a dibasic acid C10H10O6 + 2H2O, by oxidizing berberine with an alkaline permanganate, which he considered identical with hemipinic acid.
Mr. O. Bernheimer [Hlasiwetz and Gilm suggested that this substance was formed under these circumstances, and Bödeker had also previously announced that distilling berberine with either milk of lime or oxide of lead produced quinoline.] obtained as volatile products, ammonia and quinoline [Journal of the Chemical Society, March, 1884, p. 340 from "Gazzetta" 13, 342-347.] by heating berberine in a retort with five times its weight of caustic potash. The residue was found to contain two acids which in properties agree with the acids announced first by Hlasiwetz and Gilm. One of these acids was supposed by H. and G. to be protocatechuic acid, and in this connection it is well to call attention to the fact that the alkaloid Hydrastine yielded protocatechuic acid when treated by Prof. B. F. Power with excess of caustic potash. Bernheimer obtained a yellow crystalline mass by heating hydroberberine with methyl iodide in a closed tube which crystallized in the trimetric system from boiling methyl alcohol. This has the composition C20H21NO4, MeI, and when suspended in water and treated with oxide of silver produced a crystalline hydroxide of the composition C20H21NO4,MeHO+ H2O. This is strongly basic, liberating ammonia from solution of ammonium chloride. The hydroxide decomposes when heated in a scaled tube, eliminating methyl alcohol. Mr. Bernheimer concluded that hydroberberine is a tertiary base.
The same author found that by heating berberine, mythyl iodide and mythyl alcohol together a methiodide C20H17NO4, MeI, was produced.
On treating this with silver oxide, the corresponding hydroxide was formed, similar, in properties to the hydroberberine compound.
Fleitmann announced (1847) that a solution of sulphur in sulphide of ammonium; mixed with a solution of hydrochlorate of berberine, produced a brown-red precipitate of a repulsive odor. This he washed and found still to contain sulphur, but he stated that it did not yield sulphide of hydrogen by treatment with hydrochloric acid. Mr. Bernheimer, upon the contrary, found this precipitate to be decomposed under these conditions with the evolution of sulphide of hydrogen, and the result proved to be hydrochlorate of berberine free from sulphur. He therefore assumes that the precipitate is probably a persulphide of berberine. Hydriodide of berberine (C20H17NO4,HI) was produced by heating hydroberberine with iodine, both being in chloroformic solution. It is soluble in alcohol.
Salts of Berberine.—Berberine is a strong base and unites with acids, forming in many instances most beautiful crystalline salts. These salts of berberine are, as a rule, decomposed by the excess of an acid solution, especially if heated, and hence for example the muriate of berberine forms the sulphate when boiled with diluted sulphuric acid, although under like conditions the alkaloid has a stronger affinity for muriatic acid. It is obvious that the larger number of these compounds are not of interest to our readers, but a few are used in medicine and quite extensively. Some of these have interesting records, and in view of their positions in the history of the alkaloid berberine we were compelled several times to refer to them while considering that alkaloid. In this connection we will say that the early history and pharmacy of these substances has never to our knowledge been presented to the public, and we feel that in these pages it is accurately recorded for the first time.
Continued on next page.
Drugs and Medicines of North America, 1884-1887, was written by John Uri Lloyd and Curtis G. Lloyd.

