Liriodendron Tulipifera. Tulip tree.
 Parts used - Common names - Botanical description - Geographical distribution - Botanical history - Medical history and properties - Pharmacopoeial history - Commercial history - Pharmaceutical preparations - Root bark - Tree bark - Microscopic structure of the bark - Constituents
Parts used - Common names - Botanical description - Geographical distribution - Botanical history - Medical history and properties - Pharmacopoeial history - Commercial history - Pharmaceutical preparations - Root bark - Tree bark - Microscopic structure of the bark - Constituents
PARTS USED.—The inner bark of the stem and the root bark of Liriodendron Tulipifera Linn.
Natural Order Magnoliaceae, Tribe Magnolieae.
COMMON NAMES.—The name originally applied to the tree by the early settlers was Canoe Wood, from the fact that the Indians made their canoes from the tree.
When introduced into Europe it was under the name Tulip Tree, and, while that is the common name abroad, it is to be regretted that it has been little used in this country. It is very applicable, from the resemblance of the flowers to tulips, and has the sanction of all text-books, including the Pharmacopoeia, but the people do not take kindly to the name and it is but seldom used.
In the East, the tree is known as White Wood, and the early French inhabitants of the country gave it the name of Yellow Wood. In the West it is called Yellow Poplar, and in Ohio, Kentucky, etc., where the true Poplar is rare, the Tulip Tree is almost universally known simply as Poplar Tree. Writers on botany have continually protested against this name, because the tree has no relation or resemblance to the true Poplar, but the name will probably always be used. In the drug trade, and by collectors, the bark is always called Yellow Poplar Bark.
The names American Poplar, Tulip-bearing Poplar, Saddle Tree, Lyre Tree, Old Wife's Shirt (the latter three from the shape of the leaves), which are sometimes applied to the tree in books, would not here be mentioned were it not for completeness.
BOTANICAL DESCRIPTION.—The Tulip Tree is a magnificent forest tree, rising sometimes to the height of one hundred and fifty feet, and of all the deciduous trees east of the Mississippi it attains the largest dimensions, with the exception of the Sycamore (Platanus occidentalis). It reaches its greatest development in the rich, loamy, deep soil in the fertile bottoms along the rivers. [The monarch of all that we have known was destroyed by vandals in 1868. It stood on the banks of the Tradewater river, a Kentucky tributary to the Ohio, and was conspicuous even to thoughtless persons in consequence of its grandeur. A report was circulated that this old "Poplar" was a bee tree, and one Sunday morning a party of men collected, and by their united efforts, after hours of labor, felled it to the ground. Then it was discovered that it sheltered neither bees nor honey, and their only reward was the fact that this magnificent specimen of American forestry was exterminated. The diameter was said to be twelve feet at the base.] In the forests the trunk for from forty to sixty feet is unbranched and perfectly straight and erect, and almost uniform in diameter. The branches above are very regularly distributed, forming a symmetrical open head.
 The leaves (see Plate XXVI.) are of an odd shape, being somewhat of a square outline; they are nearly equally four-lobed, the side lobes separated by shallow sinuses; the terminal lobes end abruptly and as regularly as if the leaf had been folded along the mid-rib and clipped with a pair of shears. The lower lobes are generally themselves partially lobed, but the terminal lobes are usually entire. In the large leaves of vigorous shoots of young trees the lobes are narrower and separated by deep, narrow sinuses.
The leaves (see Plate XXVI.) are of an odd shape, being somewhat of a square outline; they are nearly equally four-lobed, the side lobes separated by shallow sinuses; the terminal lobes end abruptly and as regularly as if the leaf had been folded along the mid-rib and clipped with a pair of shears. The lower lobes are generally themselves partially lobed, but the terminal lobes are usually entire. In the large leaves of vigorous shoots of young trees the lobes are narrower and separated by deep, narrow sinuses.
The leaves are borne on angular, narrow foot stalks, about two or three inches long, and quiver with the least wind, and this is probably one of the reasons for calling the tree, Poplar.
The development of the leaves in the bud is peculiar. The buds are for the most part terminal and are covered externally with two thick scales cohering by their edges. These scales open, disclosing another apparent bud, covered by two scales (stipules), cohering also by their edges and forming a sheath. As the bud grows, these scales expand until they become about an inch long, when they open and disclose a young leaf and another similar bud. The leaf is bent on its petiole so that the apex points down and the young blade is folded along the mid-rib with the sides flat together (conduplicate), and lies close beside the next bud. The scales that protect the young leaves are true stipules, and after expanding they perform the office of stipules, remaining attached at the base of the leafstalk for some time, generally until the leaves have become full grown. At length the stipules fall away, leaving a circular scar on the young branch above the base of each leaf-stalk. On vigorous shoots of young branches the stipules are tardily deciduous, remaining till late in the summer.
The flowers are large and showy and appear in May and June. The tree, when in bloom, is not as conspicuous as would seem probable from the size of the individual flowers, owing to the sombre hue of these flowers and the bright green foliage which obscures them.
 The flower buds are large, and well represented in our drawing. When young they are enclosed in two obcordate scales, notched at the apex, which turn brown and fall off when the bud is about one-half the size here represented. Squirrels are very fond of these young flower buds, and often large numbers of the partly eaten buds are found under the tree, where they have fallen after having been gnawed off by squirrels.
The flower buds are large, and well represented in our drawing. When young they are enclosed in two obcordate scales, notched at the apex, which turn brown and fall off when the bud is about one-half the size here represented. Squirrels are very fond of these young flower buds, and often large numbers of the partly eaten buds are found under the tree, where they have fallen after having been gnawed off by squirrels.
 The sepals which are imbricated in the bud are three, and are reflexed in flower; they are about the length of the petals and are green, with a yellowish tinge. The petals are six, in two series; they are concave, and make a cup-shape flower, which in size and form is the same as the common single garden tulip. [When the flower is young the edges of the petals slightly lap, forming a perfect cup. As the flower becomes older they spread, as shown in our engraving, which was made from a flower past its prime.] The petals are of a fleshy texture; they are of a yellowish green color and striate or veined, and are marked on the inside near the base with a large orange red spot which is somewhat crescent-shaped, and the coloring extends irregularly up the veins.
The sepals which are imbricated in the bud are three, and are reflexed in flower; they are about the length of the petals and are green, with a yellowish tinge. The petals are six, in two series; they are concave, and make a cup-shape flower, which in size and form is the same as the common single garden tulip. [When the flower is young the edges of the petals slightly lap, forming a perfect cup. As the flower becomes older they spread, as shown in our engraving, which was made from a flower past its prime.] The petals are of a fleshy texture; they are of a yellowish green color and striate or veined, and are marked on the inside near the base with a large orange red spot which is somewhat crescent-shaped, and the coloring extends irregularly up the veins.

 The stamens are numerous. The natural number, we believe, is six, opposite each petal, or thirty-six in all, and although that is the usual number, they vary by suppression from thirty-two to thirty-six. They are an inch and a quarter long and consist each of a firm filament, one-third the length of the stamen, and of two long shallow anther-cells attached parallel to the outside of the broad filament (extrorsely adnate) [The stamens of Liriodendron being ertrorse, present a generic difference between this genus and Magnolia which has stamens all introrse.] and about two-thirds the length of the stamen, as shown in our cut (fig. 109).
The stamens are numerous. The natural number, we believe, is six, opposite each petal, or thirty-six in all, and although that is the usual number, they vary by suppression from thirty-two to thirty-six. They are an inch and a quarter long and consist each of a firm filament, one-third the length of the stamen, and of two long shallow anther-cells attached parallel to the outside of the broad filament (extrorsely adnate) [The stamens of Liriodendron being ertrorse, present a generic difference between this genus and Magnolia which has stamens all introrse.] and about two-thirds the length of the stamen, as shown in our cut (fig. 109).
The pistils are very numerous, and are attached spirally to the elongated receptacle, forming a dense conical column in the center of the flower. Each pistil Consists of a one-celled, two-ovuled ovary, a broad-winged, flat style and a minute recurved stigma.
![]() The fruit is a large cone, composed of numerous (about seventy) spirally arranged samaras, which, when mature, separate and fall away. The broad, flat wing of the samara enables it to be carried a long distance by the wind. The seeds, which in the greater number of the samaras are abortive, in the fertile fruit are one or two in number and are contained in a small cavity at the base of each samara.
The fruit is a large cone, composed of numerous (about seventy) spirally arranged samaras, which, when mature, separate and fall away. The broad, flat wing of the samara enables it to be carried a long distance by the wind. The seeds, which in the greater number of the samaras are abortive, in the fertile fruit are one or two in number and are contained in a small cavity at the base of each samara.
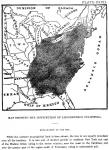 Plate XXVII. Map showing the distribution of Liriodendron Tulipifera.
Plate XXVII. Map showing the distribution of Liriodendron Tulipifera.
Explanation of the map.
While the extreme geographical limit is here shown, the tree is not equally abundant over all the territory. It is rare and of stunted growth in northern New York and east of the Hudson River, owing to the severe winters; near the coast in the Carolinas, and over the greater part of the region south of Tennessee, owing to unfavorable soil.
GEOGRAPHICAL DISTRIBUTION.—The accompanying map [By Chas. S. Sargent, published in the Report of the Forests of North America for the tenth census.] (Plate XXVII) shows the extreme limits of the Liriodendron tree. The tree is abundant in the Middle States, reaching its greatest development in the Wabash valley of Indiana, Kentucky, Tennessee, and the western slope of the Alleghenies. It is rare in the maritime regions of the Carolinas, and over the greater portions of Georgia, Alabama and Mississippi; also east of the Hudson River and the northern portions of New York.
BOTANICAL HISTORY.—We have a record of the Tulip Tree before the first settlement in America. Thomas Hariot, who accompanied Sir Richard Greenville and wrote a journal of the voyage, [Published in Latin as Part I. of De Bry's Celebrated Collection of Voyages, Frankfort on the Main, in 1590.] mentions among other economic plants of the new world, "Rakiock," referring to the Tulip tree.["Rakiock," a kind of tree so called, that are sweet wood, of which the inhabitants that were near unto us do commonly make their boats or canoes of the form of troughs, only with the help of fire, hatchets of stone and shells; we have known some so great, being made in that sort of one tree, that they have carried well twenty men at once, besides much baggage; the timber being great, tall, straight, soft, light, and yet tough enough, I think, (besides other uses) to be fit also for masts of ships."—Hariot (translated).
Pickering believes the tree "Rakiock" to be the Tulip tree, and Hariot's description of the wood and uses certainly point to this tree; however, the sentence "that are sweet wood" would not apply to it.] Again, Sir William Strachey, who arrived in Jamestown in 1612, five years after its first settlement, mentions it as Whitewood. ["The Historie of Travaille into Virginia Britannia, by Wm. Strachey" (edited by R. H. Major, and published by the Hakluyt Society in 1849). Strachey says (page 130): "Besides these fruict trees there is a white poplar," etc. Major considers this reference to apply to Populus balsamifera, but Pickering to the Tulip tree, and we have no doubt that the latter is correct.] Thus we have the history of the tree beginning with the history of the country, and it has always received more or less attention in all travels which note the objects on natural history of America. [For valuable aid in studying up the early references to the Tulip tree, we wish to acknowledge our thanks to Dr. Chas. Rice, who has kindly furnished most of our information.]
The tree is supposed to have been taken to Europe by John Tradescant, Jr., about 1656, [John Tradescant, Sr., was a German who came to London and established a museum about 1640, almost the first that was devoted to natural objects. Tradescant was not specially interested in botany, but made collections of plants for his museum, along with other objects of nature. His son, John Tradescant, Jr., was sent to America about 1650 (the precise year is not recorded) to make additions to his father's museum. He sent home a large number of new plants and trees, and it is supposed among others the Tulip tree, as it was recorded in several English gardens a few years after his return. The genus Tradescantia, which commemorates both father and son, was first applied to the American Spiderwort by Ruppius in his Flora Jenensis (1718), the plant having been introduced into the garden by Tradescant. Linnaeus adopted the name and assigned to the genus several other species.] although who introduced it is not positively known. It was cultivated, however, as early as 1663, at first as a pot-plant in hot-houses, afterwards it began to be planted in the open air, from which time it came to be extensively grown in open ground throughout Europe, and is now a common tree in that country in cultivation. At the time of Linnaeus it was well known.
In old American works on plants, and other works, when it was mentioned it was known under the generic name of Tulipifera, [Tulipifera arbor virginiana, Hermann (1687); Tulipifera caroliniana, foliis productioribus magis angulosis, Plukenett (1700), (see note, page 247 of Vol. I.); Tulipifera virginiana, tripartito aceris folio, media lacini velut abscissa. Plukenett (1700); Arbor virginiana tulipifera, Clayton (in Herb.); Tulipifera liriodendron, Miller.
The generic name Tulipifera literally means tulip-bearing, from the word tulipa and the Latin fero, to bear.
The word tulip is not a derivative from a Latin word, but from the Persian dulbend, or the related toliban, turban, in allusion to the expanded form.—C. Rice.] given to it by Paul Hermann (1687). [Paul Hermann was a German botanist and physician, who resided in the East Indies for a number of years, and afterwards returned to Europe and filled the chair of Botany in the University at Leyden from 1679 to 1695. He published the catalogue of the Leyden Garden (1687) in which the generic name Tulipifera was first given.]
Linnaeus (1737) adopted the name Liriodendron [The name is from the two Greek words λιριον a lily (a tulip), and δενδρον a tree.] for the generic name (spelling it Liriodendrun). It is to be regretted that he did not retain the old generic name, which was more appropriate. When he came to give it a specific name (1754), he selected for it the former generic name and corrected the spelling of the generic name, calling the tree Liriodendron Tulipifera, which name it has ever since retained without synonym, save Salisbury, who called it Liriodendron procerum.
Varieties.—That there are two forms of the tree, distinguished by the amount of the heart wood, was early noticed by lumbermen, and that they are distinct can not, we think, be refuted. Marshall mentions them as early as 1785. Michaux distinguishes two forms with acute and obtuse leaves, which he designated as var. acutiloba and var. obtusiloba, and Rafinesque states that the acute lobed form produces the white wood and the obtused lobed the yellow wood. Late botanical writers, however, take no cognizance of these forms, and while we are convinced that there are certainly two distinct trees as far as the color of the lumber is concerned, we have not been able to determine that they have different shaped leaves.
From an observing farmer, [Mr. Thomas Rouse, Crittenden, Ky.] who claims to know the two trees apart by the appearance of the bark, we learn the following:
The Yellow Poplar grows along streams and seems to select a damper location than the White, and the trunk is almost entirely yellow or heart wood, there being but a couple of inches of sap wood on the outside. It is very easy to split, makes good rails, and the lumber lasts a long time.
The White Poplar grows on hilly woods and dry locations, and the trunk is mostly white or sap wood. It is very difficult to split, [To use the expression of our informant, "You couldn't split it with Jersey lightning."] and decays in a few years, so it has but little value as a timber. He states that the young trees can not be distinguished to his knowledge by the bark, but that the yellow poplar bark of old trees is in long, horizontal ridges, while the white poplar bark is short and choppy.
Whether these two trees differ in other particulars than the amount of heart wood produced, or whether this is the result of the situation where they grow, remains yet to be decided.
MEDICAL HISTORY AND PROPERTIES.—Schoepf, 1787, [Materia Medica Americana, David Johann Schoepf, Erlangae, 1787, p. 90.] recognized the bark, root, leaves and seed of the Tulip tree. The bark and root in tincture as a febrifuge and rheumatic remedy; the seed as aperient; the fresh leaves to make an ointment, that was asserted to be useful in inflammations and gangrene. These were undoubtedly the uses that Schoepf found the settlers and American Indians making of the tree, for there is no record of it having been accepted by the medical profession at that clay.
Dr. J. T. Young, 1792, [American Museum, Vol. VII.] addressed a letter to Governor Clayton, of Delaware, stating as follows: "I have prescribed the Poplar in a variety of cases of the intermittent fever, and can declare from experience it is equally efficacious with the Peruvian bark. It possesses the quality of an aromatic, a bitter, and an astringent. I have never known it to fail in a single case of worms. I prescribed it to a child when convulsions had taken place, and after taking several doses, several hundred of dead ascarides were discharged."
Governor Clayton added to Dr. Young's statement that "during the late (Revolution) war Peruvian bark was very scarce and expensive, and as I was at that time engaged in considerable practice, I made a mixture of the barks of Liriodendron, Cornus florida and Quercus alba (white oak) in nearly equal quantities.). [Since both Cornus florida and white oak barks are considered by some to be of value as febrifuges, especially the Cornus, it is uncertain as to the part the Liriodendron fills in the mixture.—L.] This remedy I prescribed for several years, in every case in which I conceived the Peruvian bark necessary or proper, with at least equal, if not superior, success."
Prof. Rush, 1793, [Transactions of the College of Physicians, Philadelphia, 1793, Vol. I., pp. 183-185.] published a short notice upon its uses, stating that a mixture of Liriodendron, Cornus florida and Prinos verticillatus [Common name Black Alder; now classified by Asa Gray as Ilex verticillata.] was used by him, with as much satisfaction as any of the bitters of the shop.
B. S. Barton, 1801, [Collections for an Essay towards a Materia Medica of the United States, 1801, Part II., p. 14, (edition 1810).] states that "the bark is frequently used in intermittents. Many persons are of the opinion that, in this case, it is but little inferior to Peruvian bark. I have never employed it."
Eberle, 1822, [Treatise of the Materia Medica, Vol. I., p. 279.] devotes considerable attention to this tree, condensing extensively from preceding writers. He takes exception to the anthelmintic properties, stating, "I have given it for this purpose in several instances without deriving any good effects from it."
Chapman, 1825, [Elements of Therapeutics and Materia Medica, Vol. XI., p. 429.] adds: "The leaves are much employed in country practice as a topical application, in the headache or fever, and in sprains, bruises, painful rheumatic swellings, etc,"
Smith, 1830, [The Botanic Physician, p. 535.] states that "It is very successful in jaundice."
Rafinesque, 1830, [Medical Flora and Botany of the United States, Vol. II., p. 239.] states that "the Cherokee Indians use the leaves in poultice for sores and headache, and the ointment of the leaves for inflammation; that the seeds are laxative, and that the extract of the bark is a. useful remedy for syphilitic ulcers of the nose."
The New York edition of the United States Pharmacopoeia (1830) gave the medical properties of the drugs recognized, and, of Liriodendron tree bark, remarks, "tonic, stimulant diaphoretic. Dose one scruple to two drachms, in powder."
The first edition of the United States Dispensatory accepts Liriodendron as a "stimulant, tonic, with diaphoretic properties. It has been used as a substitute for Peruvian bark in intermittent fevers, and has proved serviceable in chronic rheumatism, dyspepsia, and other complaints, in which a gentle stimulant and tonic impression is desirable."
The early Eclectic practitioners used it but little, and this drug has never occupied a conspicuous position in their materia medica. Prof. John King gives it an important position in his Dispensatory, and Prof. J. M. Scudder, in his "Specific Medication," simply remarks, "it is a stimulant and tonic to the digestive apparatus, improving digestion and blood making. It also exerts an influence upon the nervous system, strengthening innervation and relieving those symptoms called nervous."
Thomson neglected it entirely, and we do not find it even named in his Materia Medica, or in the entire series of Thomsonian Recorders, which seems somewhat remarkable, owing to the fact that in many respects its properties coincide with those of the most valued Thomsonian remedies.
The Homoeopathic uses of Liriodendron are to be found in the special paper prepared for this publication by Prof. E. M. Hale. See page 20.
Résumé.—Liriodendron is doubtless a tonic and stimulant, and can aid digestion and sometimes relieve dyspepsia. That it has antiperiodic properties is evident, but, unless this country is debarred the more valuable Cinchona bark and its alkaloids, liriodendron will only be a domestic remedy in this respect, unless, as may be possible, its action is of a different nature. [Since writing this we have received Prof. Bartholow's physiological investigation of tulipiferine, to which the reader is directed, (on page 18).] When given in substance, there is no reason to doubt that the acrid resin as a mechanical irritant may possess some anthelmintic properties. That the drug has been neglected by the medical profession is undeniable, and careful clinical investigations should establish its exact position. We searched the United States for a physician now employing it, but, excepting Prof. Hale, without success, although we found that in domestic practice it is still occasionally used.
During the late war, the Confederate armies were compelled to resort to such substitutes for Peruvian bark and its alkaloids as were to be found in the Southern States. Liriodendron was conspicuous among the reputed antiperiodics and febrifuges, and became an important drug. Mr. Charles Mohr [Chas. Mohr is one of the most painstaking and deserving botanists of the South. Since the war he has filled several official positions, was in charge of the department of trees and woods for the last census, and is now Agent Division of Forestry (Southern), Department of Agriculture, Washington. He is also a carefully educated pharmacist.] was prominent as a Mobile pharmacist and chemist, and served some years as chemist in the Confederate Hospital department. He writes us that in the treatment of remittent and intermittent fevers, as an antiperiodic, Liriodendron bark proved very successful. [See Pharmaceutical Preparations, page 12, for the formula employed.]
PHARMACOPOEIAL HISTORY.—Liriodendron was not admitted to the first (1820) edition of the United States Pharmacopoeia. In the 1830 revision, (both Philadelphia and New York), the bark of the tree was recognized. It was carried in the secondary part, an unused drug, through succeeding revisions, until 1880, when it was discarded. There has never been an officinal preparation of Liriodendron. We have just stated in our Medical History that we could not find a record that Liriodendron is now being prescribed by any physician, and by referring to our description of the drug, it will be seen that the bark of the root is by far the richest part of the tree. Therefore we conclude that not only have the Pharmacopoeial revisors carried it unnecessarily, but have always recognized an inferior portion. [In our opinion, an object of the Pharmacopoeia is to recognize among drugs those that arc legitimate and are in use by the medical profession, regardless of their value therapeutically. That there are a number of valuable drugs unrecognized in medicine none can doubt, but, until they are accepted, it seems to us that they should not cumber oar Pharmacopoeia. Upon the other hand, it must be confessed that many inferior crude drugs and their products are extensively employed, and notwithstanding their objectionable features, we believe that our Pharmacopoeia should give them positions while they are recognized remedies.]
COMMERCIAL HISTORY.—Liriodendron bark is now sometimes employed in domestic practice as a tonic and rheumatic remedy. It appears in the drug trade in large slabs, consisting of the dried inner bark of the tree. The drug is obtained in the spring when the sap is flowing and the bark separates from the wood easily, by cutting away the outer or corky portion and then peeling off the inner bark in large slabs, generally about six inches wide and three to six feet long.
It is not used by any section of the medical profession of America. We corresponded with a number of the foremost pharmacists in each city of the United States, and in no instance did we find a record of a prescription having been received for it. An occasional call for domestic use is the only demand that is reported by pharmacists for the drug.
PHARMACEUTICAL PREPARATIONS.—In domestic practice, infusions and decoctions are sometimes administered, but the usual method is to make a spirituous tincture by covering the recent bark of the tree with whiskey. There is no recognized pharmaceutical preparation.
The formula employed in the Confederate hospitals, as recorded by Mr. Charles Mohr, of Mobile, is as follows:
Compound Fluid Extract of Poplar Bark, Dogwood and Black Willow.—
Bark of Liriodendron Tulipifera, ground.
Bark of Cornus florida, ground.
Bark of Salix nigra, ground, each fifty pounds.
Moisten with proof whiskey well freed from fusel oil by rectification through charcoal, and percolate with the same menstruum until sixteen gallons are obtained, reserving the first two gallons of the percolate. The remaining fourteen gallons are subjected to distillation at a low temperature in partial vacuo until ten gallons of spirits of twenty-two per cent, alcohol are run over. To the residuum add twenty pounds of sugar, and evaporate the syrupy liquid to thirty-four pints. Mix before cooling with the reserved tincture to make fifty fluid pints of Fluid Extract.
This extract, of the consistency of a thin syrup, is of deep brown color, at first perfectly clear, showing but a very slight turbidity after a few days' standing; it possesses in a high degree the aroma and taste of Liriodendron.—CHARLES MOHR.
ROOT BARK.—The characteristic part of this tree is the fresh inner bark of the root, although other portions of the tree resemble it in a fainter degree. The freshly broken inner bark of the root is white, and, like the inner bark of the tree, has an aromatic odor. It is intensely acrid and bitter, producing, when chewed, a painful biting sensation approaching to pepperness. Upon exposure the broken bark becomes of an orange color next to the outer bark, and this orange coloration often extends in longitudinal streaks entirely through the inner bark. When water is poured over the fresh bark, and distilled, the distillate contains a small amount of a colorless volatile oil. [Tilden states five per cent. We could only obtain it in very small proportion.] This oil has an odor somewhat aromatic, when fresh, intermediate between lemon and bergamot. Upon exposure the fragrant portion evaporates, leaving a distinct turpentine-like odor, but we could not get enough to identify. The oil is neither peppery nor bitter.
The decoction of the bark has a muddy appearance and a somewhat bitter taste, but it is not acrid. Upon standing it deposits a small amount of a buff-colored sediment. Filtration produces an amber-colored clear liquid, and upon the addition of an alkali it changes in color to red. The nitrate gives a precipitate with most alkaloidal precipitants.
Upon drying the bark, some loss of bitterness and of the sharp, pungent aromatic substances is experienced, but it would require a great length of time to render the bark entirely free from these characteristics, if they ever disappear. The bark that has been boiled with water for a considerable time, and then dried, still contains them. Prof. Maisch states [National Dispensatory, Third Edition, p. 929.] that bark collected by him and kept in a dry place for twelve years without special precaution, has still the characteristic taste in a marked degree.
TREE BARK.—The freshly broken inner bark of the tree and limbs has a white color, and a slight aromatic odor. It imparts, when chewed, at first a bitterness combined with astringency, and then a sharp, biting sensation, less marked than the root bark. [It contains very much less resin than the root hark, and the acrid nature of the bark is due to this resin.] When the breath is exhaled through the nostrils, an aromatic impression follows which is dependent upon a volatile oil. Upon exposure the broken inner bark turns of a light greenish yellow.
Distillation of the bark with water gives a liquid that contains some oily-like products, but this is destitute of the rich aroma of the fresh bark. The decoction is destitute of bitterness, and does not respond with alkaloidal tests. It contains calcium salts, an abundance of glucose, and it produces a green color with ferric chloride. The exhausted bark is destitute of bitterness, but imparts a slight turpentine-like flavor when chewed. The biting, peppery principle is destroyed by the boiling, and is neither in the distillate, the decoction nor the exhausted bark.
The leaves are slightly aromatic, of a pure bitter, but destitute of the acridity of the bark.
The mature flower buds are of a turpentine-like odor when recently broken and an aromatic turpentine-like taste, followed by bitterness. They are a favorite food for squirrels.
MICROSCOPIC STRUCTURE OF THE BARK OF LIRIODENDRON TULIPIFERA.—(Written for this publication by Robt. C. Heflebower, M. D.)—On the outside of the bark is the usual layer of cork, consisting of from four to eight cells in thickness. In this layer, the cells are flattened from without in, and on section are three or four times as long as they are wide. This layer is, in some places, thickened, so as to present an increased number of cells, being as much as twelve or fourteen cells through.
The next substance to the cork, and to the inner side of it, is the parenchyma proper of the bark. It is composed of numerous layers of cells, arranged in tolerably regular rows, parallel to the external surface of the bark. The cells of this layer are in immediate relation with the cork layer. Those cells nearest the cork are smaller and of a nearer round form than those at a greater distance from that layer.
At a short distance from the cork, parenchyma being interposed, begins the bast.
In the parenchyma are stone-elements, scattered bast fibres, resin sacs and oil cells.
The stone-cells, or stone-sclerenchyma, are seen scattered sparsely through the parenchyma.
The bast fibres or bast cells that are found in the parenchyma are exceedingly scattered, and lie in bundles of two or three cells each.
The resin-sacs are comparatively few in number, and are not of great dimensions. They occupy the place of two or three cells, are of a tolerably regular round or slightly oval form, and have no regularity of distribution. They do not lie in groups, but are solitary, and are filled with a single drop of resin, or with an aggregation of several drops. The latter is usually the case.
Oil-cells are also found in the parenchyma, though not in any great quantity. They are situated in the outer and middle portions of the parenchyma mostly, though a few are found farther in.
Extending from the cambial zone toward the cork layer, but not reaching the latter, are the bast rays. These terminate by gradually becoming smaller, until they finally end in a single cell. These rays are equivalent, both in size and position, at the cambial zone, to the wood medullary rays, and have the same general course. In this bast zone are sieve-tubes, a few stone-cells, resin-sacs, and scattered parenchyma cells with much bast fibre.
CONSTITUENTS.—Dr. Rogers, 1802, in an inaugural address, Philadelphia, among other ordinary plant constituents, identified a peculiar resin. This was the first analysis made of the bark.
Prof. John P. Emmet, 1831, [American Journal of Pharmacy, 1831, p. 5.] next made an examination of the bark, failed to find an alkaloid, but decided that the resin of preceding investigators was largely composed of a crystalline substance that he named liriodendrin. Others in common with ourselves have since failed to obtain this substance in crystalline form, and as brief abstracts only of Prof. Emmet's process are usually made, and the Journal containing his original publication is now seldom to be obtained, it is important that it should be again accurately recorded. Hence we produce it verbatim, as follows:
"The bark is to be stripped from the roots, dried, and finely pulverized; it is to be steeped for several hours in cold alcohol. The fluid, when saturated, is to be removed, and fresh portions added until the bark becomes exhausted. By employing the alcohol, hot, and straining forcibly through a fine cloth, this result will be accomplished. The alcoholic solutions are next to be filtered and transferred to a large retort, or alembic, and heated until at least two-thirds of the fluid have been recovered by distillation. Toward the end of the process, the impure liriodendrin will separate and collect at the bottom in large semi-fluid masses; it may be readily obtained by pouring out the contents of the vessel and allowing the whole to cool. The remaining liquid is then to be gently evaporated in an open vessel until it assumes the consistency of honey. The temperature should not exceed two hundred degrees.
"This dark resinous-looking mass is to be incorporated with the portion which separates during the distillation, and then triturated with a warm solution of caustic potassa, soda, or aqua ammonia. The alkali removes a large quantity of coloring matter, and th« liriodendrin, being insoluble, separates, and may be brought together by a spatula. This treatment with caustic alkali must continue until the solution passes off colorless. The liriodendrin in this state has a drab color, which becomes lighter by exposure to soft water, and a waxy lustre. At a temperature of 30° F. or 40° F. it is hard and brittle, but softens in the hand, and bears a close resemblance to putty.
"The peculiarities of this substance prevent us from obtaining it crystallized from hot concentrated alcoholic solutions. If such a solution be examined with an eye glass, it will appear saturated with exceedingly minute globules. The liriodendrin merely separates as a transparent varnish; water seems necessary for its crystallization, and in this state there is great reason for believing that it is hydrated. The water, however, should be added gradually until the color of the solution becomes pearly white, and the temperature should be as low as 40° F. or 50° F. The crystals obtained in this manner and by spontaneous evaporation are very pure, but always present different forms, among which may be observed triangles, and rhomboidal plates, interspersed with plumose or stellated prisms; some of these arc frequently limpid, while others have the micaceous appearance of boracic acid."
Emmet says that "liriodendrin in alcoholic solution is very bitter, leaving a sensation of heat upon the tongue, and burns with a brilliant white flame, giving much soot, like resins and oils. When liriodendrin is fused, it bears a close resemblance to a soft resin," and "to conclude, I may observe that the properties of liriodendrin seem to place it with camphor, as a connecting link between the resins and volatile oils."
It will be observed that Prof. Emmet is not very decided regarding the nature of the crystals, and that he finally refers the substance to the resins and volatile oils.
Griffith, 1847, [Medical Botany, 1847, p. 100. He seems not to have made it, however, but to draw his conclusions entirely from Emmet's paper.—L.] seems to doubt its nature, for he states that it can scarcely be considered as a peculiar principle, "but is rather a compound body, consisting of a resin and a volatile oil."
Tilden, 1860, [Journal of Materia Medica, 1860, p. 163.] ignores liriodendrin in his analysis, but names besides the usual constituents of plants, resins, oil, and bitter matter. He claims five per cent, volatile oil, but we, from several distillations, obtained very much smaller amounts.
Wallace Procter, 1872, [National Dispensatory, third edition, p. 929.] failed to obtain Emmet's crystals, although he obtained the resin in globules.
It seems that all commentators accept Emmet's work excepting Tilden and Procter, who made examinations of the drug, and Tilden, failing to crystallize the liriodendrin, said nothing. Hence, it is to be expected that in most works following Emmet's, the active principle of the bark will be recorded as a crystalline substance, liriodendrin. We shall refer to this subject more carefully in another place.
According to our examination, the characteristic principles, aside from the ordinary constituents of plants, are a bitter extractive, volatile oils, resin, coloring principles and an alkaloid. The aroma of the fresh bark depends upon the volatile oils; the acridity upon the resin; the bitterness (especially of the green leaves) upon the bitter extractive matter; the coloring matter and the alkaloid are not perceptible to either taste or smell.
Oleo-resin of Liriodendron.—Exhaust the undried fresh bark of the root with alcohol, spec. grav. 0.820, and distill off the alcohol, having previously added one-fourth its bulk of water. After cooling, add to the residue its bulk of water, and permit the mixture to rest for twenty-four hours in a cool location. Decant the overlying liquid, wash the resinous precipitate well with water and triturate the insoluble residue with alcohol. Filter, evaporate the alcohol, and then dissolve the residue in concentrated ether in considerable excess. Alter some days filter and evaporate the ether. By this process much of the volatile oil is lost.
Description.—This amber-colored turpentine, or oleo-resin, is sticky and viscid at ordinary temperatures, being somewhat thicker than Venice turpentine. It has a specific gravity of 1.096, an acid reaction, and the acrid taste of the bark. Upon exposure to the air for a great while in thin layer, it becomes hard and loses most of its aromatic odor.
It dissolves freely in chloroform, benzol, concentrated sulphuric ether and in alcohol, and although it is a mixture of resinous bodies and their oxidation products, it can not be disintegrated by the action of the usual solvents. We were unable to crystallize it at any temperature, or to obtain a crystalline product from it, by spontaneous evaporation or otherwise.
Solution of caustic potash dissolves it to a considerable extent, and by repeated treatments with successive portions of a hot solution it all dissolves. The addition of an acid in excess then precipitates the resin acid, with loss of its volatile oil and the aromatic odor, but with the acrid nature unimpaired. There evidently are also some decomposition products that are not at present of interest. The altered resin from the precipitated potash solution refuses to crystallize.
Cold sulphuric acid turns oleo-resin of liriodendron red, then brown; heated sulphuric acid chars it, and then effervescence follows. Cold nitric acid acts upon it very slowly; heated nitric acid decomposes it with effervescence and the production of a red liquid. Neither hot nor cold muriatic acid affects it.
Resin of Liriodendron.—This may be obtained somewhat altered by washing the oleo-resin of liriodendron with weak potash water and then dissolving the residue in sulphuric ether, filtering, and evaporating the ether. By this operation the oils are separated from the resin, which is less soluble in alkaline liquids. The resin is of a strong acrid taste, especially in alcoholic solution.
Resin of liriodendron is described by Prof. Emmet, (Liriodendrin) obtained from dried liriodendron root, as a putty like mass that crystallizes by spontaneous evaporation from alcoholic solution, to which water has previously been added until it becomes turbid. We used every endeavor to obtain crystals by this and other processes from the resin of the fresh bark, but without avail. At tunes it appeared u though we had been successful, but a critical examination demonstrated that the product was in amorphous micaceous layers; often nearly needle-like spurs appeared, transparent in thin layers, but no crystals. It could not be decolorized by animal charcoal, and neither microscopically nor otherwise, in large or small amount, could we produce a single crystal. This resin agrees in character with the crude oleo-resin, excepting that it is of somewhat firmer consistence and devoid of aromatic odor, as would seem probable from the loss of volatile oil. We are certain that only as a decomposition product, or an exceptional freak, can a crystalline resinous substance be obtained from the fresh liriodendron bark.
This resin is the "Liriodendrin" of Prof. Emmet, who has recorded in positive terms that a substance obtained by him from dried liriodendron root bark, crystallized in acicular needles and in micaceous plates. We can not dispute his testimony, but, although we obtained by his process and the use of dried liriodendron bark, his putty-like substance, time and time again, in abundant amounts, we could not crystallize it. (See page 14 for Prof. Emmet's process.) It produces a resinous body that is an alteration of the resin of the fresh bark, slightly soluble in cold alkaline solution, and of firm consistence like hard putty, of a gray, ashy color as separated from the aqueous potash solution, but of dark brownish-red from re-solution in alcohol and subsequent evaporation. It agrees with the description of Prof. Emmet excepting in formation of crystals; dissolves in alcohol, ether, chloroform and benzol, and will yield at times micaceous nodules, but, as before stated, no real crystals appeared to us. In small amount it appears transparent; in larger bulk, colored. Solutions of this resin were evaporated and examined microscopically, but no crystals could be obtained. [We must not forget, though, that some of these resinous bodies occasionally assume crystalline conditions and perhaps this may possibly crystallize, if the bark is procured at some certain season of the year.
Sometimes an impurity will produce beautiful crops of crystals. From a volatile oil of sassafras, that stood a temperature of 33° F. below zero the writer obtained large crystals of sassafras camphor by adding a speck of ordinary camphor. They remained permanent at 33° F. above zero. These crystals when melted, after some weeks, refused to crystallize until the same operation was repeated, but then again for a time, crystallized easily at 31° F. above zero.]
Prof. Virgil Coblentz made solutions of this putty-like resin, as furnished by us, and endeavored to crystallize it by Prof. Emmet's process, and otherwise, but failed. Prof. F. B. Power intended to make an ultimate analysis of this resin (liriodendrin of Emmet), but, after examination, agreed with us that it was not a definite substance and that an analysis would not be of value.
Alkaloid of Liriodendron (Tulipiferin).—The mixed watery liquid and residues from the washed resin is acidulated with hydrochloric acid, evaporated to small bulk, ammonia added to render it slightly alkaline, and the mixture agitated with sulphuric ether; the ether is decanted, and the residue is again treated with ether; the ethereal liquids are mixed, one-third their bulk of water added, and then a slight excess of hydrochloric acid; the ether is distilled, and, after cooling, the residue is filtered.
This aqueous solution still contains some impurities and should be again rendered alkaline; extracted with ether; water and hydrochloric acid added as before; the ether evaporated and the residue filtered. This will produce a colorless solution of the hydrochlorate of the alkaloid, associated with small amounts of a glucoside that is unimportant. [This glucoside can be separated by means of benzol. We could not crystallize it.]
Properties.—Colorless, odorless, tasteless, slightly soluble in water, but freely in dilute acids. Ammonia water in small amount precipitates it from aqueous solution and an excess of ammonia re-dissolves it. All the alkaloidal reagents afford precipitates with solutions of its salts, and the following reactions were recorded alike by Prof. Coblentz and ourselves:
- Potassio-mercuric iodide—White precipitate from acid solution.
- Phospho-molybdic acid—White cloudy precipitate from weak acid solution.
- Potassio-cadmic iodide—Pale yellow precipitate from acid solution.
- Picric Acid (Alcoholic solution)—Amorphous, yellow precipitate.
- Tannic Acid—Pale yellow precipitate from acid solution.
- Iodine in solution of potassium iodide—Reddish-brown precipitate.
- Platinic chloride—Light yellow precipitate
- Auric chloride—Light yellow precipitate.
- Mercuric chloride—Slight, white precipitate.
The following color tests are by Prof. Coblentz:
- Pure concentrated sulphuric acid—Yellow, changing to red.
- Fröhde's Reagent (soda-molybdate and sulphuric acid)—Bright green.
- Potassium bichromate and sulphuric acid—Green, then brown.
- Nitric acid and stannous chloride—Canary yellow.
- Sulphuric acid and nitric acid—Bright red.
Prof. Coblentz adds, "The solution furnished me was colorless and of acid reaction. Upon shaking repeatedly with benzol it was found that the associated glucoside was entirely removed. The solution of the pure alkaloid was then rendered slightly alkaline with ammonia water, shaken with absolute ether, and upon evaporation of ether it remained as an amorphous white residue which gave negative results with reagents for the glucosides.
"This alkaloid was then dissolved in various solvents, and the residues after evaporation were examined microscopically; no crystals could be obtained. The alkaloid was made into salts by means of various dilute acids, and again all attempts to obtain crystals were failures."
In estimating the amount of alkaloid, we found that but eight grains were obtained from 70,000 grains of fresh root bark. It must not be accepted that this represents the entire amount in the bark, as some of the alkaloid was lost in the purification. Probably two thirds of it escaped in this manner, and, as is usual with alkaloid makers, if these washings had been added to a succeeding batch of like amount, the product would probably have been between twenty and thirty grains.
The small amount of the alkaloid would indicate that it is unimportant, but the physiological report of Prof. Bartholow shows it to possess decided properties, and it is probable that the tonic value of the bark depends mainly upon it. The reactions that we have recorded of it are, possibly, sufficient to merit for it an individuality, and thus to increase the list of alkaloids. Although a sufficient amount was not obtained for a combustion, we believe that no described alkaloid of the related plants will agree with it, and we have therefore ventured, as advised by Prof. Bartholow, to introduce the name Tulipiferine. [The name Liriodendrine would be more appropriate, but Prof. Emmet has already applied the name Liriodendrin to a resin. In this connection we call attention to the remarks of Prof. Bartholow, on next page.] Perhaps future investigations will show that it is identical with some other alkaloid already named.
Coloring Matter of Liriodendron.—After separation of both the resin and the alkaloid, in addition to glucose and the ordinary constituents of plants, there remains in the residual liquid a peculiar coloring matter of some interest. It may be obtained and described as follows: [J. U. Lloyd in American Druggist, Wm. Wood & Co., June, I886, p. 101.]
Exhaust fresh bark of root of Liriodendron Tulipifera with alcohol, add some water and distil the alcohol. After some days filter the liquid and evaporate it to a semi-solid consistence, then incorporate this thoroughly with alcohol in considerable amount. Filter the alcohol from the extractive precipitate and add an excess of ammonia water to the filtrate. The brownish-yellow precipitate is then to be washed with alcohol and dried by spreading it upon glass and exposing it to the atmosphere. It forms brilliant deep brownish-red transparent scales that separate easily from the glass and appear very much like dark-colored scales of citrate of iron.
This substance dissolves slowly in water, producing a yellow liquid that is changed to a dark reddish-yellow by addition of an alkali. It dissolves readily in alkaline water. It is insoluble in alkaline alcohol or in benzol, chloroform or ether. Its color in alkaline aqueous solution is very much lightened by addition of excess of an acid.
It combines neither with acid nor alkali, and fails to neutralize either of them, and its precipitation from alcohol by addition of ammonia seems to be simply due to the fact that it is insoluble in such a menstruum. It is tasteless and is odorless; being of interest only from the fact that it is a characteristic coloring matter of this magnificent American tree.
Oils of Liriodendron.—When the alcoholic percolate of the fresh bark, either the root or tree, is distilled, the volatile oils pass and condense with it. This is in small amount, enough to impart an aromatic odor, and produce a turbidness when water is added, but not enough to separate.
If the fresh bark is distilled with water, the distillate contains an oil that collects upon its surface in very thin layer. Perhaps large quantities of the bark would produce enough for analysis, but lots of twenty-five pounds do not yield enough to even describe satisfactorily. This oil has at first a fragrant odor, reminding of . mixture of orange, lemon and bergamot, but upon exposure it assumes a pure turpentine-like odor. Endeavors to disintegrate the crude oil into others resulted in failures. Whether this oil is a mixture, part of which evaporates easily, leaving the other or others or whether it undergoes decomposition upon exposure, or whether polymers are formed, is yet to be decided When we consider how difficult it is to arrive at conclusions regarding ethereal oils that are produced in quantities, owing to their disposition to decompose or form polymers, we think that we can safely accept that the quantitative separation of this rare oil of liriodendron into its constituents, If it be a mixture, and its chemistry, will be a problem of the far future.
Continued on next page.
Drugs and Medicines of North America, 1884-1887, was written by John Uri Lloyd and Curtis G. Lloyd.

